

Natural Resources
Conservation Service
Ecological site R022AC200CA
High Elevation Volcanic Mountain Slopes
Accessed: 02/13/2026
General information
Approved. An approved ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model, enough information to identify the ecological site, and full documentation for all ecosystem states contained in the state and transition model.
Figure 1. Mapped extent
Areas shown in blue indicate the maximum mapped extent of this ecological site. Other ecological sites likely occur within the highlighted areas. It is also possible for this ecological site to occur outside of highlighted areas if detailed soil survey has not been completed or recently updated.
MLRA notes
Major Land Resource Area (MLRA): 022A–Sierra Nevada and Tehachapi Mountains
MLRA 22A
Major Land Resource Area 22A, Sierra Nevada Mountains, is located predominantly in California and a small section of western Nevada. The area lies completely within the Sierra Nevada Section of the Cascade-Sierra Mountains Province. The Sierra Nevada range has s gentle western slope, and a very abrupt eastern slope. The Sierra Nevada consists of hilly to steep mountains and occasional flatter mountain valleys. Elevation ranges between 1,500 and 9,000 ft throughout most of the range, but peaks often exceed 12,000 ft. The highest point in the continental US occurs in this MLRA (Mount Whitney, 14,494 ft). Most of the Sierra Nevada is dominated by granitic rock of the Mesozoic age, known as the Sierra Nevada Batholith. The northern half is flanked on the west by a metamorphic belt, which consists of highly metamorphosed sedimentary and volcanic rocks. Additionally, glacial activity of the Pleistocene has played a major role in shaping Sierra Nevada features, including cirques, arêtes, and glacial deposits and moraines. Average annual precipitation ranges from 20 to 80 inches in most of the area, with increases along elevational and south-north gradients. Soil temperature regime ranges from mesic, frigid, and cryic. Due to the extreme elevational range found within this MLRA, Land Resource Units (LRUs) were designated to group the MLRA into similar land units.
LRU "C" Northern Sierra Subalpine: Elevations are typically between 7,800 and 9,800 feet. The frost free period is between 30 and 90 days, MAAT is between 35 and 44 degrees, MAP is between 45 and 65 inches. Soils are typically cryic, but frigid soils may occur at lower elevations on southern aspects. Forests are dominated by whitebark pine (Pinus albicaulis), Sierra lodgepole pine (Pinus contorta spp. murrayana), mountain hemlock (Tsuga mertensiana) and/or California red fir (Abies magnifica).
Classification relationships
Forest Alliance = Pinus albicaulis – Whitebark pine forest; Association = tentatively Pinus albicaulis/Achnatherum californica (Sawyer, John O., Keeler-Wolf, Todd, and Evens, Julie M. 2009. A Manual of California Vegetation. 2nd ed. California Native Plant Society Press. Sacramento, California.)
Ecological site concept
This ecological site occurs in the highest elevations of the northern subalpine LRU, typically between 8,000 and 9,000 feet on mountain slopes. Slopes range from 15 to 50 percent. Soils are derived from volcanic material, and moderately deep over indurated bedrock or weathered tuff, with an loamy-skeletal particle size class. This site is dominated by sparse whitebark pine with a diverse forb understory. Cold temperatures and a short growing season limit establishment of less frost resistant conifers and shrubs, and support whitebark pine dominance. Fine textured soils developed from volcanic parent material support a diverse understory.
Associated sites
| R022AC202CA |
Shallow Andesite Ridge This ecological site occurs at lower elevations on ridges. Low sagebrush (Artemisia arbuscula) is dominant. |
|---|
Similar sites
| F022AC001CA |
Cryic Sandy Mountain Slopes This ecological site occurs on granitic soils, which have coarser textures. Whitebark pine woodlands are present. |
|---|---|
| F022AC002CA |
Cryic Sandy North Apsect Mountain Slopes This ecological site occurs on north aspects, on granitic soils. Whitebark pine- mountain hemlock forests are present. |
Table 1. Dominant plant species
| Tree |
(1) Pinus albicaulis |
|---|---|
| Shrub |
(1) Ribes |
| Herbaceous |
(1) Lupinus breweri |
Physiographic features
This site is on moderate to steeply sloping mountain sides at elevations ranging from 8,000 feet to 11,000 feet, but typically below 9,000 feet. This site can be found on all aspects, but is generally orientated on northwest to south-facing slopes which range from 15 to 50 percent.
Table 2. Representative physiographic features
| Landforms |
(1)
Mountain slope
(2) Mountain |
|---|---|
| Flooding frequency | None |
| Ponding frequency | None |
| Elevation | 8,000 – 12,000 ft |
| Slope | 15 – 50% |
| Aspect | S, SW, NW |
Climatic features
The average annual precipitation ranges from 35 to 55 inches, and falls mostly in the form of snow from November to April. The mean annual air temperature ranges from 36 to 39 degrees Fahrenheit. The frost-free (>32F) season is 15 to 60 days, and the freeze-free (>28F) season is 25 to 65 days.
Maximum and minimum monthly climate data for this ESD were generated using PRISM data (PRISM Climate Group, Oregon State University, http://prism.oregonstate.edu, created 4 Feb 2004.) and the ArcGIS ESD extract tool.
Table 3. Representative climatic features
| Frost-free period (average) | 37 days |
|---|---|
| Freeze-free period (average) | 45 days |
| Precipitation total (average) | 45 in |
Figure 2. Monthly precipitation range
Figure 3. Monthly average minimum and maximum temperature
Figure 4. Annual precipitation pattern
Figure 5. Annual average temperature pattern
Influencing water features
This ecological site is not influenced by wetland or riparian water features.
Soil features
The soils associated with this ecological site are moderately deep, and formed in colluvium and residuum derived from volcanic rock. They are well drained with moderately rapid permeability. The soil moisture regime is xeric and the soil temperature regime is cryic. Surface rock fragments smaller than 3 inches in diameter range average 35 percent cover, and larger fragments range from 0 to 5 percent. Surface textures are very gravelly peaty coarse sandy loam and very gravelly coarse sandy loam. Subsurface textures are very gravelly and extremely gravelly coarse sandy loam, and extremely cobbly sandy loam. Subsurface rock fragments smaller than 3 inches in diameter range from 38 to 48 percent by volume, and larger fragments range from 0 to 40 percent (for a depth of 0 to 40 inches). The fishsnooze and Lostridge soils are correlated to this ecological site. These soils are both Loamy-skeletal, isotic Xeric Humicryepts, but the Fishsnooze soils are moderately deep over hard andesite bedrock and the Lostridge soils are moderately deep over weathered tuff.
(This site also occurs on cool phases of the Wardcreek and Melody soils, but these components need to be updated. The Wardcreek soils are Ashy-skeletal, amorphic Xeric Vitricryands, and the Melody soils are Ashy-skeletal, mixed Lithic Vitricryands).
This ecological site has been correlated with the following mapunits and soil components in the Tahoe Basin soil survey area (CA693):
Mapunit; Mapunit name; Component; Phase; Percent
9131;Lithnip-Meiss-Hawkinspeak association, 30 to 75 percent slopes;Fishsnooze;;3
9131;Lithnip-Meiss-Hawkinspeak association, 30 to 75 percent slopes;Lostridge;;4
Table 4. Representative soil features
| Parent material |
(1)
Colluvium
–
volcanic breccia
|
|---|---|
| Surface texture |
(1) Very gravelly coarse sandy loam (2) Peaty coarse sandy loam |
| Family particle size |
(1) Loamy |
| Drainage class | Well drained |
| Permeability class | Moderately rapid |
| Soil depth | 20 – 40 in |
| Surface fragment cover <=3" | 35% |
| Surface fragment cover >3" | 5% |
| Available water capacity (0-40in) |
2.3 – 3.8 in |
| Soil reaction (1:1 water) (0-40in) |
5.1 – 6.5 |
| Subsurface fragment volume <=3" (Depth not specified) |
38 – 48% |
| Subsurface fragment volume >3" (Depth not specified) |
40% |
Ecological dynamics
Abiotic Features:
This ecological site occurs in the highest elevations of the northern subalpine LRU, typically between 9,000 and 10,500 feet on mountain slopes. Soils are derived from volcanic or metavolcanic parent material, and are shallow to moderately deep over indurated bedrock, with an ashy-skeletal particle size class. Cold temperatures and a short growing season limit establishment of less frost resistant conifers and shrubs, and support whitebark pine dominance. Fine textured soils developed from volcanic or metavolcanic parent material support a diverse understory.
Ecology-Disturbance Factors:
Individual whitebark pine trees are very slow growing, and may be up to1500 years old (Millar 2014). Stands are composed of multiple age-class single and multiple stem trees because of ongoing seedling establishment. Caching of whitebark pine seeds by Clark’s nutcracker is the primary mode of seed dispersal, with birds often caching seeds in open areas that are suitable for young seedlings. If all seeds are not consumed, they give rise to dense clusters of genetically similar whitebark pine. These clusters appear to be one tree with many stems, but are more often individual trees (Burns et al. 1990, Tomback et al. 2001a). In the absence of disturbance, ongoing recruitment from seed-caches occurs, leading to an increase in stand density over time.
Fire and avalanche are the primary natural drivers for succession. Fire ignition is frequent on these exposed ridges and mountain peaks, but there is minimal and discontinuous fuel to carry large or hot fires. Small fires may play a minor role in maintaining openings that favor the germination and survival of young whitebark pine seedlings (Burns et al. 1990, Tomback et al. 2001b, Howard 2002). Avalanche is common among the alpine peaks and ridges, and can remove swaths of vegetation in avalanche prone chutes or below wind formed cornices.
Whitebark pine forests are threatened by the non-native Cronartium ribicola, the cause of white pine blister rust (WPBR) and the native mountain pine beetle (Dendroctonus ponderosae) (Cox 2000, Tomback et al. 2001b, Howard 2002). Severe epidemics of WPBR in combination with MPB outbreaks have killed large areas of forest in the Rocky Mountains, but the whitebark pine forests in the Sierra Nevada have not suffered as high mortality. There is a complex interaction between MPB outbreaks, WPBR infection, and climate. Mountain pine beetles prefer larger diameter trees (> 6 inch diameter at breast height), as these are necessary to complete their life cycle,, and attack at the warmer, lower elevation zone of whitebark pine. Mountain pine beetles preferentially attack trees infected by WPBR. White pine blister rust will infest all whitebark pines, regardless of age or elevation (Cluck 2014).
Mountain pine beetles are a native species in North American forests, but warmer temperatures have shifted the thermal zone for mountain pine beetles upslope, subjecting higher elevations of whitebark pine to beetle attacks (Craig 2010, Keane et al. 2012, Keane and al 2013). Severe mountain pine beetle epidemics cause high mortality of overstory trees, while suppressed understory trees may be released (Meyer and Safford 2014). A flush of regeneration may occur due to the reduction in the overstory canopy providing new areas for establishment. However, the decline in seed production due to the loss of large overstory trees will leave fewer seeds to be consumed by Clark’s nutcracker and other animals which leaves fewer seeds available for regeneration, threatening stand sustainability.
The non-native WPBR was introduced into North America near Vancouver, British Columbia in approximately 1910, and has been slowly spreading across the western United States and Canada. It currently occurs throughout the Cascades, and north and central Sierra Nevada. So far, it has not been detected on whitebark pine in the southern extent of the Sierra Nevada, but has been found on a whitebark pine in Yosemite National Park and in a high Sierra location on the western slope of the Sierra National Forest (Maloney 2011). A survey was conducted in 2009 to determine WPBR presence and affect on whitebark pine survivorship in the Lake Tahoe Basin. Mean incidence of WPBR among whitebark pine populations was 35 percent, with a range of 1 to 65 percent (Maloney et al. 2012).
In order for WPBR to infect whitebark pine several synchronous phenological and environmental factors need to occur. For infection to occur in five-needled white pines, relative humidity has to be greater than 90 percent, temperatures have to be between 35.6 and 64.4 degrees F (2 to 18 degrees C), and stomates need to be open to allow WPBR entry (Maloney 2011). The basidiospores, which infect whitebark pine, are released in fall from the alternate host currants (Ribes sp.), or less commonly, lousewort or Indian paintbrush (Pedicularis or Castilleja sp.). These spores do not travel far or last long in the environment, and years with late summer or early fall precipitation are most likely when infection will occur. Whitebark pine may have early onset winter dormancy, so stomates are closed at the time WPBR basidiospores are released (Maloney 2011). The onset of winter dormancy is dependent upon the length of the growing season (temperature), precipitation and soil available water capacity (AWC).
There appears to be a relationship between soils with higher AWC and higher infection rates or intensity of stem girdling (Maloney et al. 2012). Higher soil moisture could increase WPBR mycelium growth rates and increase basidiospore production, while also allowing for whitebark pine stomates to remain open longer in the season, increasing the probability of infection (Maloney et al. 2012). This ecological site occurs on shallow to moderately deep sandy-skeletal soils, with lower AWC than the corresponding volcanic ecological site (R022AC200CA), and is likely less susceptible to WPBR infestation. This ecological site occurs on volcanic and meta-volcanic soils with finer soil textures, and thus higher AWC that the corresponding granitic ecological sites (R022AA200CA, F022AC001CA, F022AC002CA). This ecological site includes the Rifle Peak whitebark pine population, which had the highest rate of infection (65%), lowest cone production (960 cones per –H), and lowest seedling recruitment (44 seedlings per –H) in the 2009 survey (Maloney et al. 2012).
The main impact of WPBR on whitebark pine is reduction in stand cone production due to die-back of cone bearing branches from cankers girdling the branches. Mortality rates in older trees are low, and may take decades to occur. Younger trees may be killed quickly if main stem girdling causes disruption of water flow (Maloney et al. 2012). A few studies have been conducted on genetic resistance to WPBR, and results range from no resistance (Maloney, personal communication), to 26 to 47 percent in the Rocky Mountains and the Pacific Northwest (Keane et al. 2012).
Reduced seed production affects the presence and abundance of Clark’s nutcracker, and thus the number and distribution of seed caches (Tomback and Resler 2007, Keane et al. 2012). This can lead to recruitment below the threshold required to sustain populations (McKinney et al. 2009).
Predictions about climate change due to global warming suggest that the whitebark pine communities in the Sierra Nevada Mountains may be threatened by rising temperatures and precipitation changes. Recent California based climate models predict a 9 degree F increase in temperature by 2100, and broader models predict a 2 to 4 degree F increase in winter and 4 to 8 degree increase in summer (Safford et al. 2012). Models are more variable for precipitation, but local models for the Sierra Nevada, predict similar to slightly less precipitation. Most models agree that summers will become drier, since more of the precipitation is predicted to come as rain, and snow melt-off will occur earlier in spring (Hayhoe et al. 2004, Safford et al. 2012). Presently a severe drought is occurring in the Sierra Nevada, with 10 to 30 percent of average precipitation and very little snow accumulation. Whether this is climate driven, and thus will become more of the future normal remains to be seen.
High elevation areas with suitable soils and landforms for the upward migration of whitebark pine will be important for the sustainability of this community. However, in this region of the central Sierra Nevada, whitebark pine all ready occurs at the upper most elevations of the highest mountains in the area, so has little room to move upslope. The southern Sierra Nevada, with its higher mountain peaks, may prove to be an important refugium for this species.
The historic temperature range for this ecological site is between 34 to 37 degrees F. With a 2 to 6 degree warming, species such as Sierra lodgepole pine (Pinus contorta var. murrayana), or mountain hemlock (Tsuga mertensiana) may become dominant in this zone. A 9 degree warming shift over the next 85 years could make conditions favorable for upper montane species to establish. Species such as Jeffrey pine (Pinus jeffreyi) and California red fir (Abies magnifica) could survive with the longer growing season and warmer temperatures for seedling germination and leader growth. If lower elevation conifers establish in the whitebark pine zone, whitebark pine may become a seral species, dependent upon fire for continued regeneration and elimination of competitors.
The reference state consists of the most successionally advanced community phase (numbered 1.1) as well as other community phases that result from natural and human disturbances. Community phase 1.1 is deemed the phase representative of the most successionally advanced pre-European plant/animal community including periodic natural surface fires that influenced its composition and production. This phase is determined from the oldest modern day remnant forests and/or historic literature.
All tabular data listed for a specific community phase within this ecological site description represent a summary of one or more field data collection plots taken in communities within the community phase. Although such data are valuable in understanding the phase (kinds and amounts of ground and surface materials, canopy characteristics, community phase overstory and understory species, production and composition, and growth), it typically does not represent the absolute range of characteristics nor an exhaustive listing of species for all the dynamic communities within each specific community phase.
State and transition model
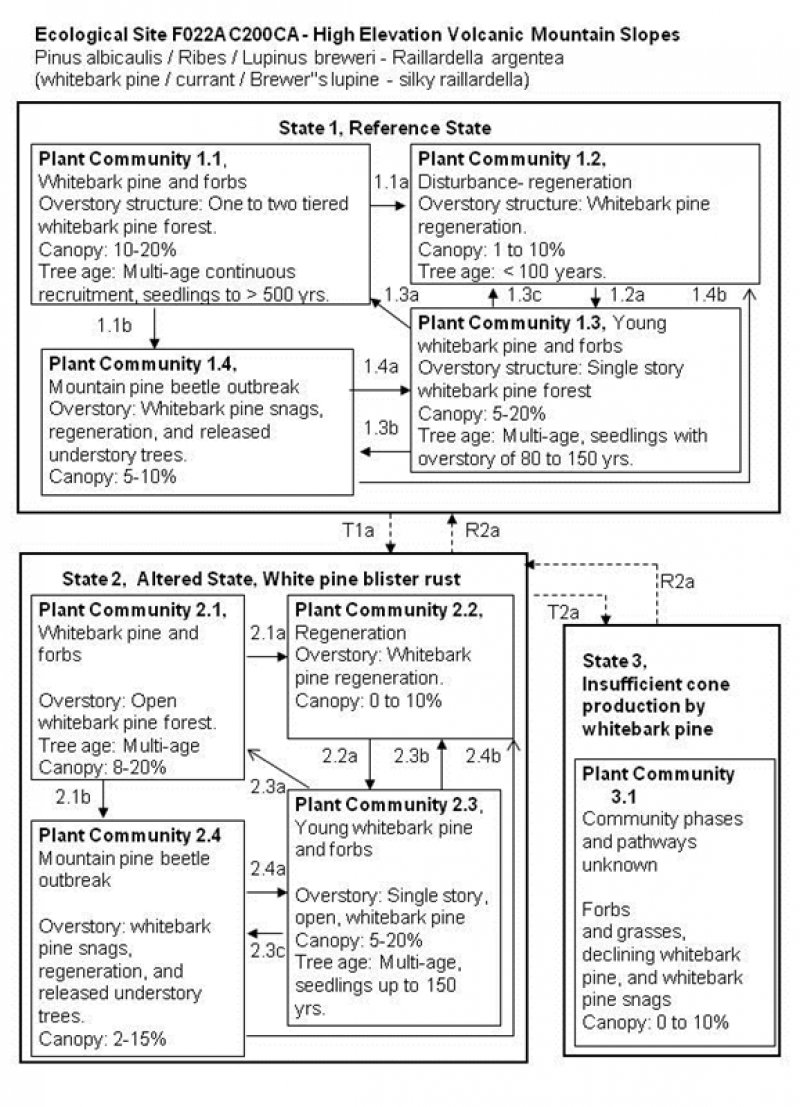
Figure 6. R022AC200CA STM
More interactive model formats are also available.
View Interactive Models
More interactive model formats are also available.
View Interactive Models
Click on state and transition labels to scroll to the respective text
State 1 submodel, plant communities
State 2 submodel, plant communities
State 3 submodel, plant communities
State 1
Reference
This state represents the reference conditions for this ecological site. This state occurs in stands of whitebark pine where WPBR is not evident, but it is limited in extent, and most of this ecological site presently exists in the WPBR altered State 2.
Community 1.1
Whitebark pine with forbs
This phase is dominated by a canopy of multiple and single-stem whitebark pines, with overstory cover ranging from 8 to 30 percent, and averaging 15 percent. Trees less than 13 feet in height range in cover from 0 to 3 percent. Overstory canopy height ranges from 15 to 30 feet with ages of 150 to 500 years old. Sierra lodgepole pine (Pinus contorta var. murrayana) and mountain hemlock (Tsuga mertensiana) may occur in limited amounts. The understory is composed of diverse forbs and currants (Ribes spp.). Cover of forbs is higher in canopy openings.
Forest overstory. This ecological site is typically a rangeland ecological site with less than 25 percent overstory canopy cover. It may develop forest structure in protected valleys or slopes, particularly in the vicinity of Mount Rose in the Lake Tahoe Basin.
Forest understory. Understory cover is relatively high with 10 to 25 percent cover. There is a high diversity of species present. Species composition is variable, but the more common species are King''s sandwort(Arenaria kingii), pioneer rockcress (Arabis platysperma), Trinity Mountain rockcress(Arabis rigidissima), dwarf alpine Indian paintbrush (Castilleja nana), Lake Tahoe draba (Draba asterophora var. asterophora), fleabane (Erigeron sp.), common woolly sunflower (Eriophyllum lanatum), marumleaf buckwheat (Eriogonum marifolium), rosy buckwheat (Eriogonum rosense), Brewer''''''''''''''''s lupine (Lupinus breweri), mountain monardella (Monardella odoratissima), spreading phlox (Phlox diffusa), California wavewing (Pteryxia terebinthina var. californica), silky raillardella (Raillardella argentea), sedges (Carex spp.), singlehead goldenbush (Ericameria suffruticosa), and gooseberry currant (Ribes montigenum). A variety of other species may be present, but have low frequency so were not included.
Figure 7. Annual production by plant type (representative values) or group (midpoint values)
Table 5. Annual production by plant type
| Plant type | Low (lb/acre) |
Representative value (lb/acre) |
High (lb/acre) |
|---|---|---|---|
| Shrub/Vine | 75 | 150 | 250 |
| Forb | 45 | 90 | 180 |
| Tree | 50 | 100 | 150 |
| Grass/Grasslike | 0 | 25 | 40 |
| Total | 170 | 365 | 620 |
Table 6. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | 0-1% | – | 0-2% | 3-15% |
| >0.5 <= 1 | 0-1% | 0-5% | 0-1% | 1-5% |
| >1 <= 2 | 0-1% | 0-5% | – | – |
| >2 <= 4.5 | 0-1% | – | – | – |
| >4.5 <= 13 | 0-2% | – | – | – |
| >13 <= 40 | 8-20% | – | – | – |
| >40 <= 80 | – | – | – | – |
| >80 <= 120 | – | – | – | – |
| >120 | – | – | – | – |
Community 1.2
Disturbance- regeneration
Fire, avalanche, or localized mortality from pathogens creates canopy gaps for whitebark pine regeneration from seed caches. Fire frequency studies are lacking for the whitebark communities in the Sierra Nevada. Mean fire return intervals for whitebark pine forest across the US range from 29 to 300 years, while moderate severity fires range from 25 to 75 years, and stand replacing fires have greater than 140 year return interval (Fryer 2002). Lightning is the main ignition source, and fires are typically small spot fires. Avalanches are common in this area, with varying frequency, size and velocities. Some avalanche chutes may have yearly or decadal avalanches in relatively confined chutes. These zones may never develop mature whitebark pine. Larger, less frequent avalanches can uproot and cause stem breakage in the avalanche path. Whitebark pine is dependent upon Clark’s nutcracker for seed dispersal. Clark’s nutcracker prefers to cache seeds in open or disturbed areas, and those that are not recovered germinate and create young tree clusters. Seed predation in normal years may be up to 97 percent, leaving few seeds for germination. Germination and seedling establishment after fire may take several years because of the high predation rate, and short dormancy period in some seeds. Years with higher summer precipitation may have higher cone yields. The cones take two years to develop. Growth of young seedlings is slow. In a typical stand, whitebark pine reaches cone maturity at 60 to 100 years (Fryer 2002). The low stature and dominance of forbs in openings away from the shade of whitebark pine, may reduce the fire severity and spread in the understory vegetation. There is very little species specific fire effect information available for these high elevation forbs and grasses. Some species may survive and resprout after low severity fire, while others will establish from on or off-site seed. Gooseberry currents do not resprout after fire, but may slowly recover from off-site animal dispersed seed. Singlehead goldenbush may increase in cover after a fire, by re-sprouting or establishment from off-site wind dispersed seed.
Community 1.3
Young whitebark pine forest
This phase is comprised of young multiple and single-stem whitebark pine trees that range in age from 80 to 150 years old. Canopy cover ranges from 10 to 20 percent. The understory is diverse with moderate cover of forbs and grasses as listed in community 1.1. There may be standing dead whitebark pine snags from previous mountain pine beetle attack or fire.
Community 1.4
Mountain pine beetle outbreak
This community phase develops after a mountain pine beetle outbreak. There is high mortality of overstory whitebark pine trees. Standing dead whitebark pine trees are dominant. Suppressed understory trees may be released by the reduction in overstory canopy. There may be a flush of regeneration in new canopy openings.
Pathway 1.1a
Community 1.1 to 1.2
Fire, avalanche, or pathogens create small gaps in canopy.
Pathway 1.1b
Community 1.1 to 1.4
Mountain pine beetle epidemic causes high mortality of overstory whitebark pine.
Pathway 1.2a
Community 1.2 to 1.3
Time, with growth and continued regeneration of whitebark pine.
Pathway 1.3a
Community 1.3 to 1.1
Time, with growth and continued regeneration of whitebark pine.
Pathway 1.3b
Community 1.3 to 1.4
Mountain pine epidemic causes high mortality of overstory trees.
Pathway 1.3c
Community 1.3 to 1.4
Disturbances such as small fires, avalanche, pathogens, or insects create openings for regeneration.
Pathway 1.4a
Community 1.4 to 1.2
Time, with growth and continued regeneration of whitebark pine.
Pathway 1.4b
Community 1.4 to 1.3
Disturbance such as fire or avalanche remove remaining canopy and initiate regeneration.
State 2
Altered State, White Pine Blister Rust
This state has developed with the introduction of the non-native white pine blister rust. The majority of this ecological site has high incidence and infection rates of WPBR, and exists in this altered state.
Community 2.1
Whitebark pine forest

Figure 8. Whitebark pine and forbs

Figure 9. Whitebark pine and forbs WPBR
This community is similar to community 1.1, but the whitebark pine has low to moderate infection rates and severity of stem girdling from white pine blister rust. There may be death of infected younger trees, and dieback of infected branches on larger trees, slightly reducing canopy cover. The understory is unaffected at this time, and is described in State 1.
Community 2.2
Regeneration- Disturbance
Regeneration occurs in canopy gaps from seeds germinating in Clark''s nutcracker caches. Overall regeneration is lower due to reduced cone production and a higher percentage of seed consumption by Clark’s nutcracker, and potential infestation and mortality of young seedlings from WPBR infection.
Community 2.3
Young whitebark pine forest
This phase is comprised of healthy young multiple and single-stem whitebark pine trees that range in age from 80 to 150 years old. There is also a percentage of WPBR infected trees, and some mortality. Canopy cover ranges from 10 to 30 percent. The understory is variable but usually sparse with subshrubs and forbs such as pioneer rockcress and spreading phlox. There may be standing dead whitebark pine snags from previous mountain pine beetle attack or fire.
Community 2.4
Mountain Pine Beetle Outbreak
This community develops after a mountain pine beetle outbreak. Whitebark pine infected by white pine blister rust may be more susceptible to mountain pine beetle attack. After an outbreak there can be high mortality of the overstory whitebark pine, leaving a stand of whitebark pine snags, and understory whitebark pine. If the infection rate of WPBR is high, this phase is high risk of transitioning to State 3.
Pathway 2.1a
Community 2.1 to 2.2
Fire, death from pathogens, insects and/or avalanches create canopy openings and niches for whitebark pine seed caches and regeneration.
Pathway 2.1b
Community 2.1 to 2.4
Mountain pine beetle outbreak causes high mortality of overstory whitebark pine.
Pathway 2.2a
Community 2.2 to 2.3
Time and growth of healthy whitebark pine.
Pathway 2.3a
Community 2.3 to 2.1
Time and growth of healthy whitebark pine.
Pathway 2.3b
Community 2.3 to 2.2
Fire, avalanche remove overstory and initiate regeneration.
Pathway 2.3c
Community 2.3 to 2.4
Mountain pine beetle outbreak causes high mortality of whitebark pine overstory.
Pathway 2.4b
Community 2.4 to 2.2
Fire or avalanche remove remaining overstory and young whitebark pine.
Pathway 2.4a
Community 2.4 to 2.3
Time and growth of unifected young whitebark pine.
State 3
Insufficient Cone Production
This state occurs, when there is insufficient regeneration of whitebark pine due to WPBR infection to maintain the stand viability. One whitebark pine stand surveyed on Rifle Peak was below the threshold cone production (Maloney et al. 2012). Cone production varies, but this area may be transitioning into State 3.
Community 3.1
Declining whitebark pine
If cone production continues to decline, and disease resistance is not found, whitebark pine will slowly decline over hundreds of years. In the absence of whitebark pine shrubs, forbs and grasses will likely become dominant.
Transition T1a
State 1 to 2
This transition is triggered by infection of whitebark pine by Cronartium ribicola, cause of white pine blister rust (WPBR), within this ecological site. WPBR affects the crown and cone producing limbs of mature trees, reducing cone production, and can kill younger trees within a year. The decrease in cone production and high mortality of young trees threatens the regenerative success of this species (Maloney et al. 2012). Repeat waves of infection by WPBR under favorable climatic conditions can worsen the situation. Reduced seed production affects the presence and abundance of Clark’s nutcracker, and thus the number and distribution of seed caches (Tomback and Resler 2007, Keane et al. 2012). This can lead to recruitment below the threshold required to sustain populations (McKinney et al. 2009).
Restoration pathway R2a
State 2 to 1
Restoration practices that have been experimented with include spraying pesticides for mountain pine beetle, and planting of hopeful, disease resistant whitebark pine.
Transition T2a
State 2 to 3
This transition occurs when WPBR infestation reduces cone production to less than 1000 cones/ Ha and basal area is < .5 m2/ acre. Below this threshold there may be insufficient seeds for dispersal by Clark’s nutcracker (McKinney et al. 2009).
Restoration pathway R3a
State 3 to 2
Restoration practices that have been experimented with include aerial spraying pesticides for mountain pine beetle, out-planting of genetically diverse whitebark pine seedlings, and potential WPBR resistant whitebark pine seedlings.
Additional community tables
Table 7. Community 1.1 plant community composition
| Group | Common name | Symbol | Scientific name | Annual production (lb/acre) | Foliar cover (%) | |
|---|---|---|---|---|---|---|
|
Forb
|
||||||
| 1 | Forbs | 45–180 | ||||
| squirreltail | ELELE | Elymus elymoides ssp. elymoides | 50–175 | – | ||
| Brewer's lupine | LUBR3 | Lupinus breweri | 10–150 | 1–8 | ||
| silky raillardella | RAAR | Raillardella argentea | 5–50 | 1–5 | ||
| King's sandwort | ARKI | Arenaria kingii | 0–30 | 0–5 | ||
| spreading phlox | PHDI3 | Phlox diffusa | 5–30 | 1–2 | ||
| mountain monardella | MOOD | Monardella odoratissima | 0–15 | 0–2 | ||
| California wavewing | PTTEC2 | Pteryxia terebinthina var. californica | 0–8 | 0–2 | ||
| pioneer rockcress | ARPL | Arabis platysperma | 0–5 | 0–1 | ||
| Trinity Mountain rockcress | ARRI | Arabis rigidissima | 0–5 | 0–1 | ||
| dwarf alpine Indian paintbrush | CANA3 | Castilleja nana | 1–5 | 0–1 | ||
| Lake Tahoe draba | DRASA2 | Draba asterophora var. asterophora | 0–5 | 0–1 | ||
| fleabane | ERIGE2 | Erigeron | 0–5 | 0–1 | ||
| common woolly sunflower | ERLA6 | Eriophyllum lanatum | 0–5 | 0–1 | ||
| marumleaf buckwheat | ERMA4 | Eriogonum marifolium | 0–5 | 0–1 | ||
| rosy buckwheat | ERRO | Eriogonum rosense | 0–5 | 0–1 | ||
|
Grass/Grasslike
|
||||||
| 2 | Grasses and sedges | 0–40 | ||||
| sedge | CAREX | Carex | 0–30 | 0–3 | ||
| western needlegrass | ACOC3 | Achnatherum occidentale | 0–10 | 0–1 | ||
|
Shrub/Vine
|
||||||
| 3 | Shrubs | 0–150 | ||||
| gooseberry currant | RIMO2 | Ribes montigenum | 0–150 | 0–5 | ||
| singlehead goldenbush | ERSU13 | Ericameria suffruticosa | 0–30 | 0–2 | ||
|
Tree
|
||||||
| 4 | Trees | 50–150 | ||||
| whitebark pine | PIAL | Pinus albicaulis | 50–150 | 8–20 | ||
| mountain hemlock | TSME | Tsuga mertensiana | 0–10 | 0–2 | ||
Table 8. Community 1.1 forest overstory composition
| Common name | Symbol | Scientific name | Nativity | Height (ft) | Canopy cover (%) | Diameter (in) | Basal area (square ft/acre) |
|---|---|---|---|---|---|---|---|
|
Tree
|
|||||||
| whitebark pine | PIAL | Pinus albicaulis | Native | – | 8–30 | – | – |
| mountain hemlock | TSME | Tsuga mertensiana | Native | – | 0–1 | – | – |
| Sierra lodgepole pine | PICOM | Pinus contorta var. murrayana | Native | – | 0–1 | – | – |
Interpretations
Animal community
Clark’s Nutcracker is the primary forager and seed disperser for whitebark pine seeds. Squirrels and other small mammals also cache seeds, to a lesser degree. Bears have been reported to raid the squirrel middens for the whitebark seeds (Howard 2002). The whitebark seeds provide valuable nutrition, and are an important food source for bears, birds, and rodents. The trees also provide cover for and nesting cavities for birds and other wildlife.
Hydrological functions
The soil associated with this site is in hydrologic group C. These soils have a slow infiltration rate when thoroughly wet as well as a slow rate of water transmission. These soils tend to have a layer that impedes downward movement of water. At this site it is bedrock that tends to obstruct the water.
Recreational uses
Hiking is the main recreation in this area, with some areas being suitable for camping. Due to the highly erodible sandy soil, trails should be constructed carefully.
Wood products
This has very low productivity and is not suited for timber or firewood production.
Other information
Re-vegetation/Restoration of disturbed areas:
The following restoration procedures are outlined in the U. S. Forest Service Fire Effects Information System (Howard 2002):
1. Assess the local extent, successional status, and vigor of whitebark pine to determine if cone crops will dwindle in the future. (Arno 1986).
2. Inventory stands to document tree age, stand structure, cone production potential, and projected time of successional replacement (Arno 1986, 1993, 1997).
3. Apply and evaluate management-ignited and wild-land for resource benefit fires designed to kill late-successional species and favor whitebark pine.
4. Conduct seed trials with white pine blister rust-resistant stock in areas where natural whitebark pine seed sources have disappeared (Arno 1986).
Supporting information
Inventory data references
The following NRCS plots were used to describe this ecological site:
MxF02h3
Ra03084 (open)
Ra03308
rx02025- Type location
Type locality
| Location 1: Washoe County, NV | |
|---|---|
| UTM zone | N |
| UTM northing | 4355951 |
| UTM easting | 246130 |
| General legal description | The site location is about 1.5 miles south of the Radio Tower, of gated forest service road 17N85, north of Mount Rose HW. Below trail about 300 feet. |
Other references
Bockino, N. K. 2008. Interactions of White Pine Blister Rust, Host Species, and Mountain Pine Beetle in Whitebark Pine Ecosystems in the Greater Yellowstone. University of Wyoming.
Burns, R. M., B. H. Honkala, and United States. Forest Service. 1990. Silvics of North America. U.S. Dept. of Agriculture For sale by the Supt. of Docs., U.S. G.P.O., Washington.
Cluck, D. 2014. Mountain Pine Beetle Outbreak in the Warner Mountains: Implications for Whitebark Pine.in Northern California Botanist Special Workshop / Session, Chico, CA.
Cox, S. 2000. Management of Whitebark Pine (Pinus albicaulis) in North American Forest and National Parks. Colorado State University.
Craig, R. K. 2010. “Stationarity is dead”—long live transformation: five principles for climate change adaptation law. Harvard Environmental Law Review:66.
Fryer, J. L. 2002. Pinus albicaulis. . In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory
Hayhoe, K., D. Cayan, C. B. Field, P. C. Frumhoff, E. P. Mauren, N. L. Miller, S. C. Moser, S. H. Schneider, K. N. Cahill, E. E. Cleland, L. Dale, R. Drapek, R. M. Hanemann, L. S. Kalkstein, J. Lenihan, C. K. Lunch, R. P. Neilson, S. C. Sheridan, and J. H. Verville. 2004. Emissions pathways, climate change, and impacts on California. Proceedings of the National Academy of Sciences 101.
Howard, J. 2002. Pinus albicaulis. Fire Effects Information System. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory
Keane, R. E., and e. al. 2013. Climate Change and Whitebark Pine: Compelling reasons for restoration. WPEF Climate Change White Paper.
Keane, R. E., D. F. Tomback, C. A. Aubry, A. D. Bower, E. M. Campbell, C. L. Cripps, M. B. Jenkins, M. F. Mahalovich, M. Manning, S. T.
McKinney, M. P. Murray, D. L. Perkins, D. P. Reinhart, C. Ryan, A. W. Schoettle, and C. M. Smith. 2012. A Wide Range Restoration Strategy for Whitebark Pine (Pinus albicaulis). Page 108 GTR RMRS-GTR-279. US Department of Agriculture, Forest Service, Rocky Mountain Research Center, Fort Collins, CO.
Korner, C., J. Paulsen, and E. M. Spehn. 2011. A definition of mountains and their bioclimatic belts for global comparisons of biodiversity data. Alpine Botany.
Maloney, P. 2011. Incidence and distribution of white pine blister rust in the high elevation forests of California. Forest Pathology:8.
Maloney, P., D. R. Vogler, C. E. Jensen, and A. D. Mix. 2012. Ecology of whitebark pine populations in relation to white pine blister rust infection in subalpine forests of the Lake Tahoe Basin, USA: Implications for restoration. Forest Ecology and Management 280:166-175.
McKinney, S. T., C. E. Fiedler, and D. F. Tomback. 2009. Invasive pathogen threatens bird–pine mutualism: implications for sustaining a high-elevation ecosystem. Ecological Applications 19:10.
Meyer, M. D., and H. D. Safford. 2014. Effects of Mountain Pine Beetle Outbreak on Whitebark Pine Stand Structure, Inyo National Forest, California.in Northern California Botanist Special Workshop / Session, Chico, CA.
Millar, C. I. 2014. Climate, Bark Beetles, and High Elevation Pines (Whitebark and Limber) in the Great Basin: Not Always a Bad Combination. .in Northern California Botanist Special Workshop / Session, Chico, CA.
Millar, C. I., R. D. Westfall, D. L. Delaney, J. C. King, and L. J. Graumlich. 2004. Response of subalpine conifers in the Sierra Nevada, California, USA, to 20th-century warming and decadal climate variability. Arctic, Antarctic, and Alpine Research 36:181-200.
Safford, H. D., M. North, and M. D. Meyer. 2012. Climate change and the relevance of historical forest conditions. Page 22 in M. North, editor. Managing Sierra Nevada Forest. United States Department of Agriculture.
Tomback, D. F., S. F. Arno, and R. E. Keane. 2001b. Whitebark Pine Communities: Ecology & Restoration. Island Press, Washington D.C.
Tomback, D. F., and L. M. Resler. 2007. Invasive pathogens at alpine treeline: consequences for treeline dynamics. Physical Geography 28:397-418.
Rangeland health reference sheet
Interpreting Indicators of Rangeland Health is a qualitative assessment protocol used to determine ecosystem condition based on benchmark characteristics described in the Reference Sheet. A suite of 17 (or more) indicators are typically considered in an assessment. The ecological site(s) representative of an assessment location must be known prior to applying the protocol and must be verified based on soils and climate. Current plant community cannot be used to identify the ecological site.
| Author(s)/participant(s) | |
|---|---|
| Contact for lead author | |
| Date | |
| Approved by | |
| Approval date | |
| Composition (Indicators 10 and 12) based on | Annual Production |
Indicators
-
Number and extent of rills:
-
Presence of water flow patterns:
-
Number and height of erosional pedestals or terracettes:
-
Bare ground from Ecological Site Description or other studies (rock, litter, lichen, moss, plant canopy are not bare ground):
-
Number of gullies and erosion associated with gullies:
-
Extent of wind scoured, blowouts and/or depositional areas:
-
Amount of litter movement (describe size and distance expected to travel):
-
Soil surface (top few mm) resistance to erosion (stability values are averages - most sites will show a range of values):
-
Soil surface structure and SOM content (include type of structure and A-horizon color and thickness):
-
Effect of community phase composition (relative proportion of different functional groups) and spatial distribution on infiltration and runoff:
-
Presence and thickness of compaction layer (usually none; describe soil profile features which may be mistaken for compaction on this site):
-
Functional/Structural Groups (list in order of descending dominance by above-ground annual-production or live foliar cover using symbols: >>, >, = to indicate much greater than, greater than, and equal to):
Dominant:
Sub-dominant:
Other:
Additional:
-
Amount of plant mortality and decadence (include which functional groups are expected to show mortality or decadence):
-
Average percent litter cover (%) and depth ( in):
-
Expected annual annual-production (this is TOTAL above-ground annual-production, not just forage annual-production):
-
Potential invasive (including noxious) species (native and non-native). List species which BOTH characterize degraded states and have the potential to become a dominant or co-dominant species on the ecological site if their future establishment and growth is not actively controlled by management interventions. Species that become dominant for only one to several years (e.g., short-term response to drought or wildfire) are not invasive plants. Note that unlike other indicators, we are describing what is NOT expected in the reference state for the ecological site:
-
Perennial plant reproductive capability:
Print Options
Sections
Font
Other
The Ecosystem Dynamics Interpretive Tool is an information system framework developed by the USDA-ARS Jornada Experimental Range, USDA Natural Resources Conservation Service, and New Mexico State University.
Click on box and path labels to scroll to the respective text.