

Natural Resources
Conservation Service
Ecological site F094DY002WI
Poor Fens
Last updated: 3/29/2024
Accessed: 03/04/2026
General information
Approved. An approved ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model, enough information to identify the ecological site, and full documentation for all ecosystem states contained in the state and transition model.
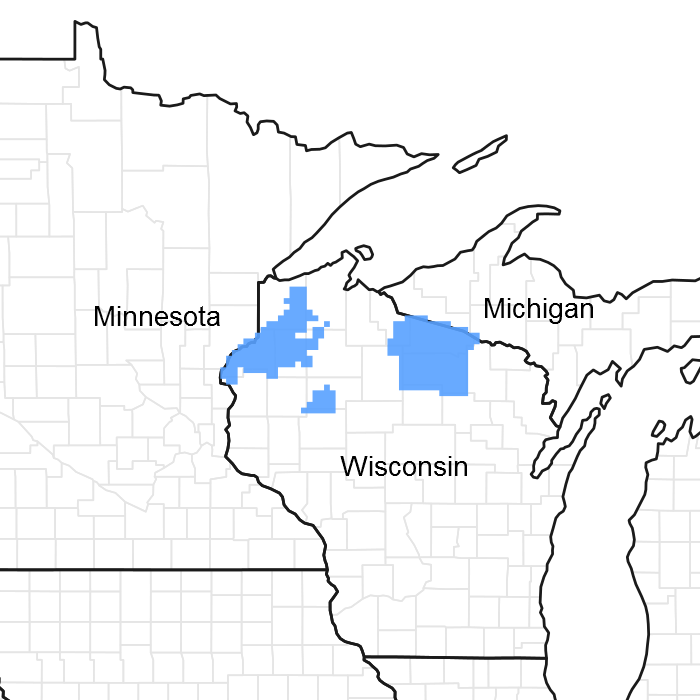
Figure 1. Mapped extent
Areas shown in blue indicate the maximum mapped extent of this ecological site. Other ecological sites likely occur within the highlighted areas. It is also possible for this ecological site to occur outside of highlighted areas if detailed soil survey has not been completed or recently updated.
MLRA notes
Major Land Resource Area (MLRA): 094D–Northern Highland Sandy Pitted Outwash
The Northern Highland Sandy Drift region (also referred to as MLRA 94D) lies mostly in northern Wisconsin with a few narrow outwash channels extending into the upper peninsula of Michigan. MLRA 94D encompasses 1.364 million acres and is surrounded by much larger, geologically different MLRAs. MLRA 94D is characterized mainly by sandy and gravelly soils formed in outwash sediments deposited by melt-water streams from late Wisconsin-Age glaciers, which receded from the area about 10,000 years before present (Attig 1985). The glacial deposits in MLRA 94D have been measured up to 280 feet (85 meters) thick; they overlie a dome of crystalline, igneous bedrock that is one to two billion-years old. This ancient bedrock, which is highly resistant to erosion, gives the region a prominent elevation - accounting for the Northern Highland name - despite the overall subdued landscape relief. Subdued relief is due mainly to repeated Pleistocene glaciations as well as hundreds of millions of years of prior geologic erosion. There are, however, several prominent areas within 94D that have bedrock outcrops and shallow to bedrock, extremely bouldery soils.
The Northern Highland also has the distinguishing feature of numerous water bodies which cover about 14% of the MLRA surface area. Wetlands are also abundant; they are mostly peat-filled and occupy about 33% of the surface area of the Northern Highland according to Soil Survey data. A wide variety of vegetation types exist in these wetlands, ranging from emergent aquatic species (including culturally important wild rice beds) to thickly forested swamps and bogs. Climatic and topographic conditions—cold, wet and low relief—in this region are highly conducive to the formation of extensive peatlands (Heinselman 1963). The lakes and wetlands are the result of depressions in the landscape left by the glaciers. The Poor Fens ecological site occupies about 80,000 acres of MLRA 94D.
Classification relationships
US Forest Service Subsection—Northern Highland Pitted Outwash (212Xb): This subsection has nearly identical boundaries with MLRA 94D and is similarly named. The Forest Service ecological classification system (ECOMAP 1993), and the database behind it, becomes increasingly valuable to NRCS information needs when the primary land use is forest and recreation, as is the case in MLRA 94D. The Northern Highland MLRA/subsection consists of eight distinct Landtype Associations (LTAs; Map 2). LTAs are landscape-scale ecological units based on geology, soils, vegetation and landform. These 8 LTAs show that landscape diversity is found even within a small, relatively homogeneous MLRA. The Forest Service has developed a nested hierarchy of ecological units, from large regions down to individual sites. NRCS Ecological Site Descriptions (ESDs) coincide with finest level of resolution in the Forest Service system; referred to as Ecological Landtypes (ELT) and Ecological Landtype Phases (ELTP). However if Ecological Sites are conceptual thus essentially dimensionless, then the size and scale of resolution of ES as recognized on the ground is highly variable. Sites can be small (ex. vernal pools 10 feet in diam.) or large (ex. homogeneous peatlands exceeding 1,000 acres). Poor Fens are recognized and defined as ecological sites by several different authorities on the subject and they occur in several other MLRAs. As such, we recognize the variability inherent in this ecological unit.
Ecological site concept
Poor Fen ecological sites in MLRA 94D are non-forested wetlands, although woody plants (scattered trees and sparse to dense shrub thickets) are common in some parts of these sites. Poor Fens are similar to rich fens in that they have a greater amount of groundwater flow-through than forested wetlands (Rydin and Jeglum 2013). However, Poor Fens have a lower concentration of mineral plant nutrients supplied by their groundwater inputs. The soils in Poor Fens formed in peat derived mainly from reeds and sedges overlying sandy mineral sediments. These soils are low in mineral plant nutrients and the pH of the soil is highly acidic, typically below 5.0. Because of the sandy and acidic nature of the glacial deposits in this region, near-surface groundwater does not contain enough calcium ions to produce calcareous rich fens. However, relative to the other peatland ecological sites in this region, these sites are medium in plant nutrients. Productivity is reduced on this site due to the extended root zone hydroperiod. Frequent but temporary ponding is commonplace and permanent ponds often exist within or adjacent to these sites. Typically, a high lateral flow rate of water through the root zone prevents complete anoxia. The hydroperiod is long enough to prevent the site from being colonized by trees. Mosses, non-vascular plants which also lack a root system—tend to dominate the wettest areas. Sedges, rushes and grasses are dominant on most of the area of these sites. There are some woody species which are adapted to very wet conditions (e. g. leatherleaf—Chamaedaphne calyculata); they have root systems that can form an intertwined network above the water table. Tangled, near surface root systems catch and hold organic debris, which leads to development of a hummock and hollow micro-topography commonly found on organic soils (Crum 1988). Groundwater flow-through on these sites supplies enough mineral nutrients to increase species richness.
Associated sites
| F094DY001WI |
Peat Bogs There are four non-floodplain, peatland ecological sites in this MLRA. Most organic soil map units in this MLRA have a mix of these sites. They are differentiated by cover type. Poor Fens represents the non-forested sites. Although the Peat Bog ecological site has a muskeg phase on which the forest cover is greatly reduced, this phase can be distinguished from Poor Fens by moss thickness greater than 1 foot. |
|---|---|
| F094DY003WI |
Mucky Peat Swamps Poor Fen ecological sites occur as narrow, moat-like areas between the Mucky Peat Bog ecological sites and mineral uplands. |
| F094DY004WI |
Mucky Peat Bogs Besides the moat effect on the edge of Mucky Peat Swamp ecological sites, Poor Fens occur as areas where woody vegetation has been drowned-out within Mucky Peat Swamps. |
Similar sites
| F094DY015WI |
Wet Loamy-Mantled Depressions Mucky Floodplains and Poor Fens have similar vegetation, both are non-forested, graminoid-dominated sites. However, the Mucky Floodplain sites lack the moss-derived surface layer common to Poor Fen sites. |
|---|
Table 1. Dominant plant species
| Tree |
Not specified |
|---|---|
| Shrub |
(1) Alnus incana |
| Herbaceous |
(1) Carex stricta |
Physiographic features
The Poor Fen ecological site in MLRA 94D occurs mainly in ice-block depressions or in broad, low-gradient drainageways on pitted outwash plains, sandy moraines and in some cases ephemeral glacial lake basins. These sites are often adjacent to lakes or ponds. On some lake-margins, they are essentially floating mats of vegetation. These sites may also be part of the headwaters of streams and rivers, but they are not found downstream on floodplains. Flooding is an important ecological process that produces hydrologically different ecological sites. Poor Fen sites in this region can be part of vast wetland complexes that includes forested swamps and bogs, and open water marshes. Thus the largest of Poor Fen ecological sites may encompass hundreds, and in a few cases, thousands of acres. Slopes range from 0 to 2 percent. On the largest sites, a one mile long continuous slope of just one percent creates hydraulic head of over 50 feet. A hydraulic head of this magnitude causes very rapid flow-through as well surface runoff.
Elevation in this MLRA ranges from a low of 1,390 feet above sea level where the Wisconsin River exits the southwestern edge of the MLRA (section 20, T. 33 N., R. 6 E.) to maximum of 1,872 feet above sea level on a steep sandy ridge in the northeastern part of the region (near the southeast corner of section 9, T. 42 N., R. 9 E.). These elevational differences cause a regional flow of water toward the south. However, local variation in upland slope gradient, length and aspect all contribute a great deal to the ecological variability of wetlands (Boelter and Verry 1977).
Nevertheless, due to the lack of woody vegetation, it can be deduced that Poor Fen ecological sites receive a proportionally larger amount of groundwater plus runoff from upland sources than forested wetland ecological sites (assuming equal precipitation across the region). This implies that Poor Fens are downslope from a larger watershed than forested wetlands (again assuming other factors are equal). Under this scenario, the quantity of inflow and outflow is enough to flush nutrients from the site. On the other hand, discharge from Poor Fens is occasionally impeded by high water levels in nearby water bodies. Lake levels, which may also be artificially manipulated, typically undergo considerable natural variation on sandy substrates. This is a geologically young landscape dominated by porous sandy soils, lakes and peatlands. Many hydraulic connections exist from surface water to groundwater through the highly permeable soils and sediments. Despite the abundance of surface water, the surface drainage network is not well developed compared to older landscapes. Even so, two major rivers, the Wisconsin and the Flambeau, have their headwaters in this region. This signifies the abundance and importance of water on this landscape. Poor Fen ecological sites are a result of both local and regional hydrology, implying that changes in either may affect this site.
There are three main watershed subunits in this region. The upper Wisconsin River watershed drains about 66% of the MLRA and this includes the source of the Wisconsin River at Lac Vieux Desert (elev. 1683 ft.). The North and South Forks of the Flambeau River drain the northeastern one-third of the MLRA. The source of the South Fork is Round Lake in Price County, WI and the North Fork has its source at the has its source at the confluence of the Manitowish and Bear Rivers upstream from the Turtle-Flambeau Flowage (elev. 1562 ft.) in Iron County, WI . Storage and steady release of water from peatlands to rivers and lakes maintains their navigability and supports their aquatic ecosystems (Boelter and Verry 1979; WI DNR 2014).
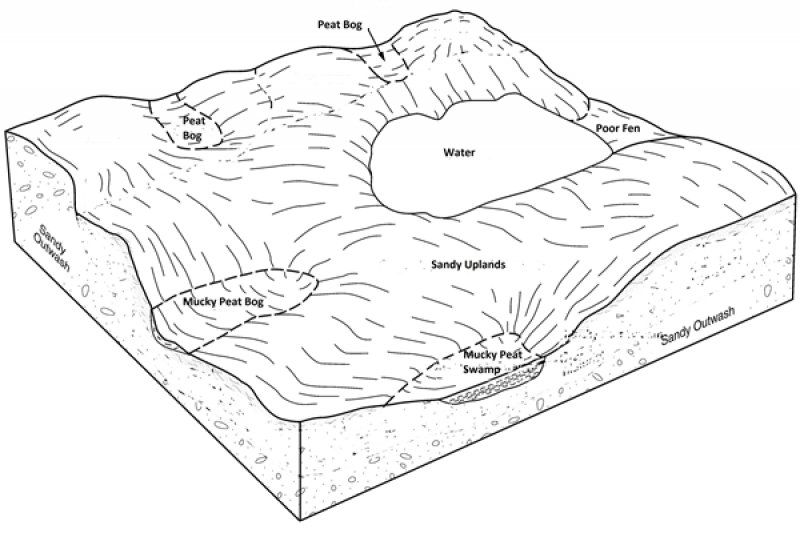
Figure 2. peatland ecological sites on pitted outwash
Table 2. Representative physiographic features
| Landforms |
(1)
Fen
(2) Depression (3) Drainageway |
|---|---|
| Flooding frequency | None |
| Ponding duration | Brief (2 to 7 days) to very long (more than 30 days) |
| Ponding frequency | Occasional to frequent |
| Elevation | 1,390 – 1,872 ft |
| Slope | 2% |
| Ponding depth | 2 – 12 in |
| Water table depth | 12 in |
| Aspect | Aspect is not a significant factor |
Climatic features
The climate is humid continental with very cold winters and warm summers. As is common across northern Wisconsin, two-thirds of the precipitation falls as rain during the relatively short growing season of late May to early September. Most of the rainfall is transpired by plants. Snow cover is likely in the months of November through April. Snow cover prevents deep frost penetration which promotes groundwater recharge. Also, some species of wildlife such as ruffed grouse (Bonasa umbellus) and snowshoe hares (Lepus americanus) benefit from deep snow, while others, such as whitetail deer (Odocoileus virginianus) and wild turkeys (Meleagris gallopavo) are hindered by deep snow. April and October are the prime months for groundwater recharge. PRISM data shows that the average minimum and maximum temperatures are increasing and average precipitation is decreasing for this area; this trend is predicted to continue and will likely have significant ecological consequences (WICCI Report 2012).
Although the seasonal temperature variations and growing season precipitation are relatively uniform across this MLRA, there are three factors that introduce variation across this region and in particular, the wetlands of the region. The first is the “Lake Superior snow-belt” that reaches into the northern edge of this region. The snow-belt is an area of extra winter precipitation that increases both spring runoff and flow downstream. The second is the phenomenon of cold air drainage that occurs in areas of high relief or in areas with numerous landscape depressions, as is the case in this region. Cold air drainage occurs because of differential heating of air masses on a local basis. Colder air is denser, thus heavier than warmer air and flows to lower elevations (much like water), eventually collecting in depressions. This effectively lowers the growing degree days and shortens the frost-free period within depressions as compared to uplands, thus wetland plants must become adapted to a shorter growing season.
The third factor is the tendency for exposed south-facing slopes to be warmer and drier due to increased solar radiation. Although slope is much less a factor in peatlands, a similar effect of is found in Poor Fens when they are exposed to solar heating and there is no dispersing wind. This produces a volume of air in the Poor Fen that is warmer than the surrounding uplands. Typically this reverses at night, the heat radiates away and the Poor Fen becomes cooler than the surrounding area. The effect of these localized temperature differences is that these non-forested depressional sites are more frequently subject to temperature extremes than upland sites. The net effect of microclimatic factors in Poor Fens (frost pockets, localized temperature extremes, increased exposure to snow, ice and wind) can inhibit tree growth through frost-kill of seedlings, damage from snow, wind and ice and reduced respiration during temperature extremes (Kozlowski and Pallardy 2002).
The effect of wind is another weather phenomenon that is localized by landform. In Poor Fens, there are few if any natural windbreaks—such as trees, hills and ridges. The cooling and drying effect of wind may at times mediate against temperature extremes but may also desiccate some plants and select for those adapted to drought stress as well as excess wetness. Also, the proximity of these sites to open water serves to increase the fetch of wind. This may account for the fact that of the few trees found in Poor Fens none get very tall due to wind damage or windthrow. Mitchell‘s (2013) review of wind disturbance in forests indicates that wet soils combined with exposure to wind present maximum windthrow hazard.
Median precipitation in this region typically supplies enough water for annual ponding on these sites. Interestingly, many wetland plants have adaptations for drought stress (leaves that are small but thick and waxy-coated, i.e., schlerophyllous leaves, fewer stomata, and fibrous root systems). Stress is induced by the lack of oxygen in the root zone, which impairs root functions, specifically the uptake of water and nutrients. Thus many wetland plants, particularly ericaceous shrubs, have schlerophyllous leaves which slow water loss. Many graminoid species (reeds, sedges and grasses) have very narrow leaves which lessens water loss. In addition, they develop aerenchyma tissues that supply oxygen to the roots to maintain function in ponded conditions. These adaptations mitigate the physiological drought conditions imposed by hypoxia in wetland soils.
Table 3. Representative climatic features
| Frost-free period (characteristic range) | 85-104 days |
|---|---|
| Freeze-free period (characteristic range) | 122-134 days |
| Precipitation total (characteristic range) | 30-32 in |
| Frost-free period (actual range) | 78-106 days |
| Freeze-free period (actual range) | 115-138 days |
| Precipitation total (actual range) | 30-32 in |
| Frost-free period (average) | 94 days |
| Freeze-free period (average) | 128 days |
| Precipitation total (average) | 31 in |
Figure 3. Monthly precipitation range
Figure 4. Monthly minimum temperature range
Figure 5. Monthly maximum temperature range
Figure 6. Monthly average minimum and maximum temperature
Figure 7. Annual precipitation pattern
Figure 8. Annual average temperature pattern
Climate stations used
-
(1) RHINELANDER [USC00477113], Rhinelander, WI
-
(2) EAGLE RIVER [USC00472314], Eagle River, WI
-
(3) MINOCQUA [USC00475516], Minocqua, WI
-
(4) REST LAKE [USC00477092], Manitowish Waters, WI
Influencing water features
Water is integral to virtually all of the ecological processes on this site. Poor Fen sites can be classified as Ground-water Depressional using the Hydrogeomorphic Model (Brinson 1993); although in appearance, they have much in common with Organic Flats. However there are Poor Fen sites that intergrade to both Lacustrine-fringe and Sloping Wetland sites. The fluvial nature of these sites is pronounced, however, they are not channelized, water flows through them in a sheet-like fashion. Generally, ground-water inputs to any wetland are more mineral rich than surface-water inputs (runoff and direct precipitation), unless there is accelerated soil erosion occurring in the watershed resulting in sedimentation and subsequent degradation of wetlands. Peatlands are notably dependent on mineral nutrient inputs from outside the wetland (Rydin and Jeglum 2013). However, the uplands in this region are sandy, low in weatherable minerals, and acidic. As such, they discharge groundwater that reflects those conditions. Thus, they provide a lower level of dissolved nutrients than more base-rich parent materials, which is compounded in Poor Fens by the dilution factor of excess inflows. By contrast, there are richer wetland sites in this region, which are typically forested, indicating somewhat lesser water inflows and a shorter saturated hydro-period, and thus less dilution of nutrients in solution. Also, forested wetlands have a much more effective drainage outlet, which, in the case of the most species-rich and productive forested sites, is a small headwater stream. Using this model, all wetlands in MLRA 94D can be classed by the influence of two main factors: 1) the predominant source of water—groundwater or precipitation—which determines the mineral nutrient constituents of the water, and 2) closed or open drainage—which defines the effectiveness of the discharge outlets which in turn affects the hydroperiod of any given wetland (Novitzki 1982). Increased residence time of water in a wetland decreases oxygen and increases acidity. There is an interplay of these factors that determines the nature of a wetland. Moreover, large wetlands are often complexes of two or more wetland types (plus possible intergrades). Extragrade sites would include flood-prone sites which must be considered separately due to significantly different hydrologic processes. Every wetland site has ecological properties that are governed by the parameters of the water budget and Darcy’s Law of saturated hydraulic conductivity. In statement form, the water budget for a wetland equation is: the sum of all inflow sources equals sum of all outflow discharges. Wetlands discharge to the atmosphere through evapotranspiration and potentially to surface water or to the groundwater. Inflows to wetlands are from the atmosphere through direct precipitation, from the ground through base flow and from the surface via overland runoff or channeled flow. Poor Fens have the highest rates of groundwater inflow and outflow of the four peatland ecological sites recognized in the area thus far. Darcy’s law takes many forms, but fundamentally, it incorporates variables such as hydraulic head and saturated hydraulic conductivity (Ksat) to predict flow rates through soils.
Many of the lakes in this region are darkly stained by naturally-occurring, soluble organic acids derived from peatlands. This phenomenon affects lake ecology by causing lake water to warm faster in spring and reducing light penetration thereby altering aquatic vegetation.
In summary, all wetland sites can be ordinated along two main environmental gradients. Those two gradients are: 1) the chemical gradient imposed by the mineral nutrient levels and pH of inflows and 2) the hydrological gradient which is reflected in the hydroperiod of the site (Rydin and Jeglum 2013). The results of these influential hydrological and chemical gradients are most readily observed through the various vegetative responses. Ponding and emergent aquatic vegetation occur when inflow rate consistently exceeds outflow rate, herbaceous vegetation results from frequent temporary ponding and woody vegetation predominates on sites that have a frequently aerated root zone. Many of the forested wetland sites in this region have an identifiable channeled outlet, Poor Fens typically do not.
Soil features
The soils on this ecological site are Histosols, they formed in decomposing plant remains. The upper 6 inches are peat, which is partly decomposed moss. The next layer is muck with thin layers of mucky peat, greater than 45 inches thick. And the underlying substratum is composed of sandy mineral sediments. The pH of the organic layers is 4.5 or greater. This site is low to medium in soil fertility. Other important features of the organic soils on this ecological site include: 1) they are slow to warm up in spring, thus shortening the growing season 2) they are frequently but briefly ponded, thus the water stays somewhat oxygenated 3) because of lack of woody vegetation, the surface is less hummocky than other peatland ecological sites; and 4) the most common soil map unit components are Carbondale (Euic, Frigid, Hemic Haplosaprists), Seelyevile (Euic, Frigid, Typic Haplosaprists) and Markey (Euic, Frigid, Terric Haplosaprists).
Table 4. Representative soil features
| Surface texture |
(1) Peat |
|---|---|
| Family particle size |
(1) Sandy |
| Drainage class | Very poorly drained |
| Permeability class | Moderately slow to moderately rapid |
| Soil depth | 80 in |
| Surface fragment cover <=3" | Not specified |
| Surface fragment cover >3" | Not specified |
| Available water capacity (0-40in) |
10 – 15 in |
| Calcium carbonate equivalent (0-40in) |
Not specified |
| Electrical conductivity (0-40in) |
2 mmhos/cm |
| Sodium adsorption ratio (0-40in) |
Not specified |
| Soil reaction (1:1 water) (0-40in) |
4.5 – 5 |
| Subsurface fragment volume <=3" (Depth not specified) |
Not specified |
| Subsurface fragment volume >3" (Depth not specified) |
Not specified |
Ecological dynamics
The information in this ecological site description (ESD), including the state-and-transition model (STM), was developed using historical data, professional experience, and scientific studies. The information is representative of a complex set of plant communities. Not all scenarios or plants are included. Key indicator plants, animals, and ecological processes are described to inform land management decisions.
Vegetation on Poor Fen sites is, for the most part, governed by water quantity and quality (Zwieg and Kitchens 2009; Venterink et al. 2001). Relay floristics (i.e. succession), which is so common on upland sites, is not prevalent in wetlands (Kotar and Burger 2009). However, there is a great deal of ecological facilitation (positive feedback) between plant species in stressful environments and this leads to changes in vegetation over time (Bertness and Hacker 1994). For example, the growth of shrubs provides woody root systems that create hummocks which are newly created habitat for plant species that would not thrive without the hummocks.
Disturbance factors, other than altered hydrology, mainly inhibit the growth of the sparse trees that grow on the site (Kozlowski and Pallardy 2002, Cohen and Kost 2008). Even herbivory, which one might speculate would be important in a graminoid dominant community, is not important on these sites. The various herbaceous plant communities found on these sites are mainly a reflection of the range of hydrologic and chemical gradients. More water causes less woody biomass (mainly shrubs) production and more abundant obligate hydrophytes (such as Scirpus, Carex and Juncus species as well as bryophytes); less water provides favorable habitat for tree and shrub growth. In general, due to the massive amount of wind and animal dispersed seed, trees are ubiquitous in this region. For trees not to be present on a site, a controlling factor must be operating. Excess wetness serves that function on Poor Fens. The chemical gradient also determines which species are present, but even nutrient-poor sites can produce forests populated by species adapted to those conditions such as black spruce and tamarack, however slow-growing they may be. Nutrient-poor sites have fewer species overall than nutrient-rich sites. Those depauperate sites also have stress-tolerant species dominating the understory (e. g., Sphagnum spp. and Chamaedaphne calyculata). Poor fens often have two or three dominant plant species, but also have a long list of species that occur in lower frequencies and abundances. This may be due to the influence of micro-sites within Poor Fens; some micro-sites may pond more often and some may be better aerated. Thus micro-sites increase biodiversity by providing habitat for aquatic species (like bladderworts and pickerel-weed) in wetter locations and woody species (willows, alder, and possibly some trees) on drier locations. Given the volume of inflow to these sites, the hydraulic pathways off this site must be efficient. Discharge to a subsurface aquifer is restricted due to a commonly occurring subsurface aquitard in organic soils, also evapo-transpiration is variable with growing conditions, and therefore discharge to surface water is the most likely outflow pathway. Within Poor Fens in general, groundwater inflow rate and discharge rate to surface water are the key water budget variables. Again, they are called Poor Fens because of a paucity of cationic plant nutrients (Ca+2, K+, NH4+) and bicarbonate ions (HCO3-) arriving on-site from groundwater and other inflow sources (Chapin et al. 2004).
Disturbance on Poor Fen ecological sites largely involves an altered hydroperiod: too much or too little water. Both natural and man-made factors contribute to those disturbances. Simply put, too much water converts the site to aquatic habitat, and too little water promotes the growth of woody vegetation. Under either disturbance regime, unwanted invasive species may find habitat. People have been manipulating water levels in lakes, rivers and flowages in this area since the logging era in the late 1800’s; this was done for power supply and for transportation purposes. Since then, water levels have also been managed for water supply, flood control, wildlife and fisheries, and recreational purposes. Each of the 146 dams (source: http://dnr.wi.gov/topic/Dams/data.html) in this MLRA has the potential to permanently inundate some wetlands and consequently create new lake-margin ecological sites. Roads through wetlands unintentionally alter wetland hydrology by forcing more ponding in some areas, and more drainage in others. Being non-forested wetlands, these sites are not subject to common large-scale forestland disturbance factors (e.g., wind, ice, fire, insects, and diseases).
State and transition model
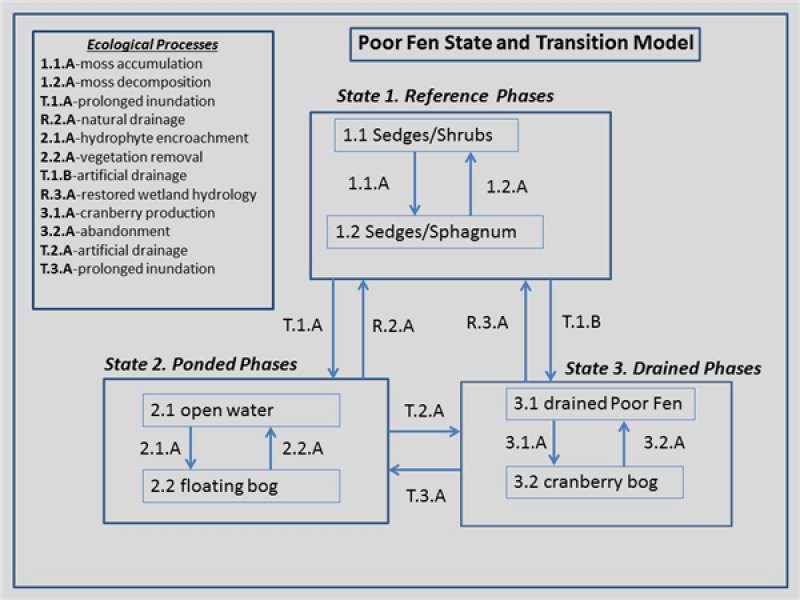
Figure 9. Poor Fen STM
More interactive model formats are also available.
View Interactive Models
More interactive model formats are also available.
View Interactive Models
Click on state and transition labels to scroll to the respective text
Ecosystem states
State 1 submodel, plant communities
State 2 submodel, plant communities
State 3 submodel, plant communities
State 1
Reference Phases
There are two main community phases of Reference State 1 for the Poor Fen ecological site, a Sedges/Shrubs phase (1.1) and a Sedges/Sphagnum phase (1.2). Despite the fen hydrology, both are nutrient-limited plant communities, they are especially low in calcium and potassium ions. The mineral uplands that contribute groundwater to this site are low in base-forming cations and rich in iron and aluminum which promote acidic conditions. The fen-like characteristics of this site are due to the flushing action of groundwater flow-through; this prevents a build-up of highly acidic conditions found within Peat Bog ecological sites. This excess of water on the site leads to a mainly treeless condition, except on slightly higher and thus drier micro-sites. The cycle between two main phases is largely governed by the speed with which water passes through the system and effects on oxygen availability. Highly oxygenated water and fluctuating water tables give rise to phase 1.1 Sedges-Shrubs. Circumstances like downstream obstructions or zones of reduced hydraulic conductivity slow the overall flow rate, increasing the opportunity for biological activity to decrease oxygenation below the tolerance for trees and shrubs. This promotes phase 1.2 Sedges-Sphagnum. If mosses continue to accumulate, the site may eventually convert to a Sphagnum lawn, i.e. a predominantly moss community (Crum 1988). Nevertheless, if the groundwater-dominant hydrology remains in force the Sphagnum-dominated site will remain a Poor Fen.
Community 1.1
Sedges-Shrubs

Figure 10. Poor Fen reference site: Sedges-Shrubs Phase

Figure 11. Swamp milkweed on Poor Fen reference site
Aeration in the root zone promotes the growth of woody plants. Shrubs are a component in this phase because they are conspicuous on parts of the ecological site, not because they are dominant across the entire site. Most woody plants lack aerenchyma or gas conducting tissue which is an adaptation to permanently wet sites. On the other hand, many herbaceous wetland species oxygenate their roots using aerenchyma. In this phase, the shrubs that are present are those adapted to short periods of saturation-induced anoxia. Common shrubs include alder (Alnus incana), willows (Salix species), bog birch (Betula pumila) and various Spiraea, Cornus (dogwood) and Viburnum species on less acidic sites, with ericaceous subshrubs (leatherleaf and Vaccinium species) indicative of more acidic sites. Trees may occur as sporadic individuals where moisture conditions permit. They are mostly tamarack with an occasional black spruce, paper birch or red maple, there are species with shallow and extensively lateral root systems. The presence of alder often indicates thinner than average organic soil layers. The most abundant sedge species are Carex stricta (tussock sedge), Carex lacustris (lake sedge), Carex lasiocarpa (wiregrass sedge) and Carex aquatilis (water sedge). Also important are Calamagrostis canadensis (Canada bluejoint), Scirpus species (bulrushes, wool-grass) and Juncus species (rushes). Broadleaf herbaceous species include swamp milkweed and pickerel weed (pictured in Photos 1 and 2); other distinctive flowering plants include northern bog goldenrod (Solidago uliginosa), Joe-Pye weed (Eutrochium maculatum), northern blue-flag(Iris versicolor), bog bean (Menyanthes trifoliate), swamp loosestrife (Decodon verticillatus) and swamp candles (Lysimachia terrestris).
Forest overstory. Even though Poor Fens are a non-forested ecological site, trees are occasionally present in small patches. This can be considered an inclusion of another ecological site within the Poor Fen. If there is more than one tree in any given 20 by 20 meter area, it can be considered an inclusion of a forested ecological within the Poor Fen.
Forest understory. The Poor Fen species list is derived from recorded field observations and published sources: NRCS habitat type inventory data; Forest Service ecological inventory data; State and County cover type data; Curtis (1971) for ordination of plant communities and floristics; Kotar et al. (2010) for Habitat Type and floristics; Black and Judziewicz (2009) for wildflowers and their habitat; Crum (1992) for mosses and their habitat; Hipp (2008) for sedges and their habitat; Judziewicz et al. (2014) for grasses and their habitat.
Table 5. Ground cover
| Tree foliar cover | 0-1% |
|---|---|
| Shrub/vine/liana foliar cover | 1-10% |
| Grass/grasslike foliar cover | 50-90% |
| Forb foliar cover | 5-20% |
| Non-vascular plants | 10-30% |
| Biological crusts | 0% |
| Litter | 1-10% |
| Surface fragments >0.25" and <=3" | 0% |
| Surface fragments >3" | 0% |
| Bedrock | 0% |
| Water | 5-90% |
| Bare ground | 0% |
Table 6. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | 0% | 0-1% | 20-30% | 5-10% |
| >0.5 <= 1 | 0% | 0-2% | 20-30% | 5-10% |
| >1 <= 2 | 0% | 0-2% | 20-30% | 5-10% |
| >2 <= 4.5 | 0% | 0-2% | 5-15% | 1-5% |
| >4.5 <= 13 | 0-1% | 0-2% | 0% | 0% |
| >13 <= 40 | 0-1% | 0% | 0% | 0% |
| >40 <= 80 | 0% | 0% | 0% | 0% |
| >80 <= 120 | 0% | 0% | 0% | 0% |
| >120 | 0% | 0% | 0% | 0% |
Community 1.2
Sedges-Sphagnum

Figure 12. Poor Fen reference site: Sedges-Sphagnum Phase
This phase represents the increase in Sphagnum moss found in consistently wet, weakly minerotrophic environments. This phase develops because the mix of environmental conditions allows mosses to persist but not to dominate the site. Sedges will likely maintain species richness but their biomass will be lower in this phase. The most important sedge species are the smaller and more slender Carex lasiocarpa (wiregrass sedge) and C. disperma (two-seeded sedge). Also increasingly important are Eriophorum species (the various cotton grasses).
Forest overstory. This phase typically has no tree species present.
Forest understory. This phase typically has 90% to 100% moss cover of various species with vascular plants growing up through the moss cover.
Table 7. Ground cover
| Tree foliar cover | 0% |
|---|---|
| Shrub/vine/liana foliar cover | 0-1% |
| Grass/grasslike foliar cover | 50-90% |
| Forb foliar cover | 10-20% |
| Non-vascular plants | 90-100% |
| Biological crusts | 0% |
| Litter | 0-5% |
| Surface fragments >0.25" and <=3" | 0% |
| Surface fragments >3" | 0% |
| Bedrock | 0% |
| Water | 5-90% |
| Bare ground | 0% |
Table 8. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | 0% | 0% | 1-5% | 2-10% |
| >0.5 <= 1 | 0% | 0% | 1-5% | 2-10% |
| >1 <= 2 | 0% | 1-5% | 20-80% | 2-10% |
| >2 <= 4.5 | 0% | 0% | 5-10% | 2-10% |
| >4.5 <= 13 | 0% | 0% | 0% | 0% |
| >13 <= 40 | 0% | 0% | 0% | 0% |
| >40 <= 80 | 0% | 0% | 0% | 0% |
| >80 <= 120 | 0% | 0% | 0% | 0% |
| >120 | 0% | 0% | 0% | 0% |
Pathway 1.1.A
Community 1.1 to 1.2


Moss accumulation: A variety of mosses will grow on this site depending nutrient content of the groundwater entering the site. In general, calcium ions are toxic to Sphagnum spp. but other types of mosses are more tolerant (Rydin and Jeglum 2013). A high volume of groundwater plus surface water inflow dilutes the concentration of calcium ions, creating more favorable conditions for Sphagnum. Lesser inflows allow for woody vegetation to survive, which can shade out the mosses. Moss accumulation is indicative of both lower nutrient and wetter conditions. The resulting mix of moss species on Poor Fen sites is highly correlated to calcium ion content. The calcium ion (Ca+2) content of poor fen groundwater is relatively low (about 10 ppm) compared to richer sites, but somewhat higher than Peat Bog sites, on which Sphagnum flourishes. This allows for the growth of Sphagnum species in a mix with other moss species. Over time, this produces phase 1.2. This phase may be a stage in the long-term trend toward ombrotrophication in peatlands. This occurs when organic material accumulates to the point where groundwater does not influence the nutrient status of the root zone, and Sphagnum predominates, as in Peat Bogs. Essentially, Poor Fen sites have resisted ombrotrophication because they drain effectively.
Pathway 1.2.A
Community 1.2 to 1.1


Moss decomposition: Mosses are not favored when in competition with vascular plants in areas of oxygenation and nutrient enrichment, also Sphagnum is not very shade tolerant. Therefore, mosses persist but they do not to overwhelm vascular plants in Poor Fens. Moreover, they decompose at the same rate as vascular plants (Scheffer et al. 2001). In addition, a wide variety of decomposers (fungi, bacteria, protozoa and multicellular fauna) will be more functional in richer environments (Rydin and Jeglum 2013). Thus, a thick peat layer derived from moss is an indication of a sunny but nutrient-poor environment. In a typical Poor Fen, the growth and decay of mosses reaches and maintains a steady state. Dead moss plants will decay at rate that prevents a build-up of a peat moss layer. However in shaded areas, under shrubs for example, the growth rate of Sphagnum is less than the rate of decay of dead moss plants. Thus, drought cycles or any hydrologic perturbation that decreases wetness can increase moss decomposition by favoring the encroachment of shrubs.
State 2
Ponded Phases
The ponded state is relatively common on this site; however the duration is usually less than an entire growing season. Brief ponding occurs during spring snowmelt and after heavy rains. Short duration ponding followed by significant lateral flow seldom results in vegetation change. However, ponding that lasts longer than one growing season will likely cause significant vegetation changes (Weltzin et al. 2000). Emergent aquatic species (such as cattails and bulrushes) will be favored initially followed by submerged aquatic plants in deeper areas. Woody species will be drowned-out but remain as standing dead trees or shrubs thus indicating former moisture conditions. Large wetlands often have naturally-occurring surface micro-topographic and elevational changes across the site; these conditions produce long-term ponded areas interspersed with short-term ponded areas. Temporary pools of various durations tend increase plant and animal diversity of a site(Rydin and Jeglum 2013). Also, waterfowl, furbearers, reptiles and amphibians, some fishes and numerous arthropods can benefit from well designed and maintained man-made impoundments within wetland complexes. On the negative side, inadvertent ponding may kill valuable upstream timber; and in general, reduce habitat for some rare native species such as swamp-pink orchids (Arethusa bulbosa) and possibly cause the spread of invasive or less desirable species such as purple loosestrife. By maintaining natural wetland hydrology, which included ponded areas, we are investing in ecosystem maintenance and fostering vital ecosystem services.
Community 2.1
Open Water

Figure 13. Poor Fen: Ponded Phase
The open water phase can be colonized by a variety of aquatic plants; some are extremely valuable to wildlife such as native cattails or are culturally significant to people, such as wild rice is for Native Americans. The two main types of herbaceous aquatic plants are 1) submergent species—found in deeper water, which includes plants in genera Potamogeton (pond weeds), Utricularia (bladderworts) and Myriophyllum (milfoils)—including the highly invasive Eurasian water-milfoil and curly pondweed; and 2) emergent species—found only in shallower water (i. e. less than 2 meters deep; bulrushes, burr-reeds, spike rushes and cattails are the most common). There are no woody plants growing on the submerged sites; but standing dead timber leftover from previous conditions may be present. Dead trees standing in water, singular or in groups provide potential nest sites for wood ducks (Aix sponsa) or great blue herons (Ardea herodias) and fishing perches for belted kingfishers (Megaceryle alcyon) and bald eagles (Haliaeetus leucocephalus). Typically, the low nutrient status and low temperatures prevents these waters from becoming choked with vegetation.
Table 9. Ground cover
| Tree foliar cover | 0% |
|---|---|
| Shrub/vine/liana foliar cover | 0-1% |
| Grass/grasslike foliar cover | 10-20% |
| Forb foliar cover | 10-20% |
| Non-vascular plants | 1-5% |
| Biological crusts | 0-1% |
| Litter | 0-2% |
| Surface fragments >0.25" and <=3" | 0% |
| Surface fragments >3" | 0% |
| Bedrock | 0% |
| Water | 100% |
| Bare ground | 0-1% |
Table 10. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | 0% | 0-1% | 1-5% | 1-5% |
| >0.5 <= 1 | 0% | 0-1% | 1-5% | 1-5% |
| >1 <= 2 | 0% | 0-1% | 1-5% | 1-5% |
| >2 <= 4.5 | 0% | 0-1% | 1-5% | 0-1% |
| >4.5 <= 13 | 0% | 0% | 0% | 0% |
| >13 <= 40 | 0% | 0% | 0% | 0% |
| >40 <= 80 | 0% | 0% | 0% | 0% |
| >80 <= 120 | 0% | 0% | 0% | 0% |
| >120 | 0% | 0% | 0% | 0% |
Community 2.2
Floating Bog
On open water margins, plants can exist on floating mats of tangled roots and entrapped debris. Sometimes through wave or frost action these floating mats of vegetation can break free of any attachment to dry land and literally float away from shore. They float along until they run aground elsewhere and then start growing at the new location. Mosses, sedges and even some small shrubs and trees are capable of surviving these nautical excursions.
Table 11. Ground cover
| Tree foliar cover | 0% |
|---|---|
| Shrub/vine/liana foliar cover | 5-10% |
| Grass/grasslike foliar cover | 20-30% |
| Forb foliar cover | 5-15% |
| Non-vascular plants | 20-40% |
| Biological crusts | 0% |
| Litter | 0-5% |
| Surface fragments >0.25" and <=3" | 0% |
| Surface fragments >3" | 0% |
| Bedrock | 0% |
| Water | 10-20% |
| Bare ground | 0-5% |
Table 12. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | 0% | 1-5% | 2-5% | 2-5% |
| >0.5 <= 1 | 0% | 1-5% | 2-5% | 2-5% |
| >1 <= 2 | 0% | 2-5% | 5-10% | 2-5% |
| >2 <= 4.5 | 0% | 2-5% | 5-10% | 2-5% |
| >4.5 <= 13 | 0% | 0% | 2-5% | 0% |
| >13 <= 40 | 0% | 0% | 0% | 0% |
| >40 <= 80 | 0% | 0% | 0% | 0% |
| >80 <= 120 | 0% | 0% | 0% | 0% |
| >120 | 0% | 0% | 0% | 0% |
Pathway 1.A
Community 2.1 to 2.2
Hydrophyte encroachment: As plant detritus increases to the point where deposits are above the water in shallow areas, terrestrial hydrophytes become dominant. Carex, Scirpus and Juncus species are common. Floating mats of vegetation begin to form over the water surface when woody subshrubs colonized the edges and form tangled roots that trap organic debris. Over time, floating bogs form and they are held together by root masses and they are buoyant enough to create an oxygenated root zone.
Pathway 2.A
Community 2.2 to 2.1
Vegetation removal: Vegetation removal in wetlands typically does not require a DNR permit unless there is soil disturbance or material is deposited in the wetland. Federal law prohibits wetland conversion to farmland if the farm operator is enrolled in USDA programs such as crop insurance or commodity price supports. Cranberry producers in Wisconsin have a blanket exemption from DNR individual permit requirements for cranberry bed construction and supporting activities.
State 3
Drained Phases
The drained state is mainly an artificial creation. In contrast to other regions of the Midwest where wetlands were drained for agriculture on a large scale, this MLRA was not, largely due to short growing seasons and acidic soils. Drainage in this area are mostly related to construction projects. Wetlands are protected by law but highway construction is an exception; many roads cross wetland sites and each one changes the local hydrology somewhat. Constructing a roadbed across these sites perpendicular to groundwater flow inevitably causes a drier downstream side and a wetter upstream side, vegetation will respond accordingly to these disturbances (Hribljan 2012). Agricultural drainage on these sites is a major construction project in itself. Well-maintained systems of ditches, dikes, water control structures and functional outlets are needed to effectively lower the water table on these sites. These efforts were abandoned many years ago, but in some cases, the remnants remain and are plainly visible. Farming may have been tried, but currently commercial cranberry bogs are the only viable agricultural use of these sites. Another drainage scenario involves climate change. Prolonged drought may have effects similar to drainage, such as native species extirpation and peatland subsidence (Hribljan 2012).
Community 3.1
Drained Poor Fen
These sites are occasionally drained for development of some kind, e.g., road-building or cranberry production. Sometimes incidental drainage occurs in the course of a project on another site, such dam removal or repair. Natural drainage can occur when severe drought occurs, ideally fen hydrology is restored when precipitation increases. Under drought conditions, woody vegetation is likely to increase. In the case of on-site development, a certain amount of organic soil material is removed and with it, goes the native plant community. The excavated material is largely biomass that can be composted and used as a soil amendment. Or if it mostly moss, it can be used for horticulture purposes in potting mixes. There is currently no commercial peat harvesting in Wisconsin, but there is a limited amount of moss harvesting by permit. Reclaiming peat harvested areas in Michigan and Minnesota has been slow and ecosystem services are not likely to be completely restored.
Forest overstory. Drainage allows these sites to be colonized by trees, quaking aspen is a copious seed producer which are blown in on the wind. This species is ubiquitous in the area and spreads to any available site.
Forest understory. Drainage opens these sites to invasive or exotic species such reed canary grass, Phragmites, and purple loosestrife. Reed canary grass is well established in the area, Phragmites is starting to enter the area from the east and purple loosestrife is spreading from the south.
Table 13. Ground cover
| Tree foliar cover | 0-5% |
|---|---|
| Shrub/vine/liana foliar cover | 5-15% |
| Grass/grasslike foliar cover | 30-60% |
| Forb foliar cover | 20-30% |
| Non-vascular plants | 1-5% |
| Biological crusts | 0-5% |
| Litter | 1-5% |
| Surface fragments >0.25" and <=3" | 0% |
| Surface fragments >3" | 0% |
| Bedrock | 0% |
| Water | 0% |
| Bare ground | 1-5% |
Table 14. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | 0-1% | 1-5% | 1-5% | 1-5% |
| >0.5 <= 1 | 0-1% | 1-5% | 5-10% | 1-5% |
| >1 <= 2 | 0-1% | 1-5% | 10-20% | 5-15% |
| >2 <= 4.5 | 0-1% | 1-5% | 20-30% | 5-15% |
| >4.5 <= 13 | 0-1% | 1-5% | 0% | 0% |
| >13 <= 40 | 0-1% | 0% | 0% | 0% |
| >40 <= 80 | 0% | 0% | 0% | 0% |
| >80 <= 120 | 0% | 0% | 0% | 0% |
| >120 | 0% | 0% | 0% | 0% |
Community 3.2
Cranberry Bog

Figure 14. Cranberry beds and supporting land-from point A to point B

Figure 15. Cranberry harvest
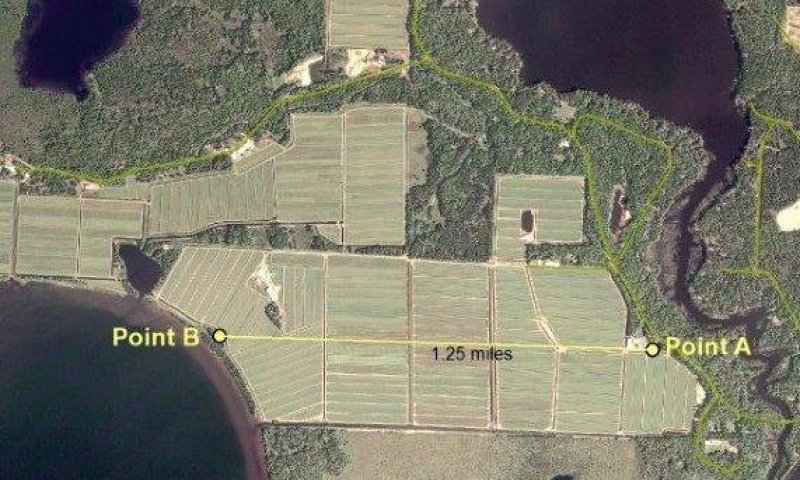
Figure 16. Aerial view of cranberry beds-point A to point B
Commercial cranberry production in Wisconsin started in the late 1800s. State laws give growers the right to use any amount of water they need for cranberry production (www.wiscran.org). There are about 2000 acres in commercial cranberry beds in MLRA 94D; mostly in the vicinity of Tomahawk, Three Lakes and Manitowish Waters, WI. Most of those beds are on converted Poor Fen ecological sites. In addition, land development supporting cranberry production such as roads, ditches, borrow pits, sheds and equipment yards occupy about another 8,000 acres adjacent to the cranberry beds; this developed land is often adjacent to and has an ecological impact on undeveloped Poor Fen ecological sites. More cranberry bog development can be predicted for the future because on a per acre basis, cranberries are consistently the highest value crop grown in Wisconsin (www.wiscran.org).
Figure 17. Annual production by plant type (representative values) or group (midpoint values)
Table 15. Ground cover
| Tree foliar cover | 0% |
|---|---|
| Shrub/vine/liana foliar cover | 90-95% |
| Grass/grasslike foliar cover | 0-5% |
| Forb foliar cover | 0-5% |
| Non-vascular plants | 0-1% |
| Biological crusts | 0-1% |
| Litter | 0-1% |
| Surface fragments >0.25" and <=3" | 0% |
| Surface fragments >3" | 0% |
| Bedrock | 0% |
| Water | 1-5% |
| Bare ground | 1-5% |
Table 16. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | 0% | 90-95% | 1-5% | 0-5% |
| >0.5 <= 1 | 0% | 0% | 1-5% | 0-5% |
| >1 <= 2 | 0% | 0% | 0% | 0% |
| >2 <= 4.5 | 0% | 0% | 0% | 0% |
| >4.5 <= 13 | 0% | 0% | 0% | 0% |
| >13 <= 40 | 0% | 0% | 0% | 0% |
| >40 <= 80 | 0% | 0% | 0% | 0% |
| >80 <= 120 | 0% | 0% | 0% | 0% |
| >120 | 0% | 0% | 0% | 0% |
Pathway 1.A
Community 3.1 to 3.2
Cranberry production: Cranberry production is an important economic activity in this MLRA; and currently it is the only form of agriculture on Poor Fen ecological sites. As of 2008, cranberry growers in Wisconsin are exempt from the wetland protection measures administered by the US Army Corp of Engineers. The extremely high cost of developing productive cranberry beds (in excess of $30,000/acre) prevents excessive development. If market conditions warrant, there is probably room for more cranberry bog development in this region.
Pathway 2.A
Community 3.2 to 3.1
Abandonment: There are several reasons for cranberry bog abandonment, such as disease problems, economic conditions, dike system failure, conversion to another land use like peat harvesting. At present, there is no commercial peat harvesting in MLRA 94D (WI DNR 2014). Nevertheless, peat can be harvested from these sites. In the past, individuals have used peat resources for their own purposes. Practical uses for peat resources include: soil amendments for the home gardener, potting soil material for greenhouse operations, and absorbent material for industry. Based on the large extent of peatlands in MLRA 94D, development pressure is not overwhelming. Since it is now widely recognized the important role peatlands play in the hydrologic cycle (Zedler 2000), peat harvesting is not likely to be encouraged; especially considering that the current recreation and tourism-based economy of this region depends on the ecosystem services supplied by peatlands relative to water supply and water quality.
Transition 1.A
State 1 to 2
Prolonged inundation: the transition from a seasonally ponded to a continuously ponded state is caused by either natural or artificial means. In pre-settlement times, beaver (Castor canadensis) dams were the most common cause (Cohen and Kost 2008). In some areas, beavers have returned and resumed dam building. Beavers are attracted to flowing water, so not every Poor Fen site is subject to their activity. However beaver dams can back up water for a considerable distance and beavers are diligent in maintaining their structures, so the upstream effects of beaver dams may not be readily apparent on the ground. In the post-European settlement era, man-made impoundments most the common cause of prolonged inundation and they are typically very large (Hribljan 2012). A system of dams, dikes and weirs on creeks, rivers and lakes maintains their water level. In this region, Powell Marsh, the Manitowish chain of lakes, the Eagle River chain of lakes, the Three Lakes chain of lakes, the Rainbow Flowage, the Willow Flowage, the Turtle-Flambeau Flowage, Lake Alice, the Hat Rapids Flowage, Boom Lake and the Rhinelander Flowage all have artificially maintained water levels that have inundated thousands of acres of lake-margin Poor Fens. Also, inadvertent ponding may be caused by poorly designed road-building, poorly maintained or abandoned drainage systems, or catastrophic flooding (Hribljan2012).
Transition 1.B
State 1 to 3
Artificial drainage: transition from a natural fen drainage pattern to an artificially drained condition would require constructing deep ditches with effective outlets. Ineffective outlets lead to water in ditches that doesn’t flow. Some ditches outlet to rivers, but most outlet to lakes. High water levels in the outlet water body will undoubtedly back up water into the ditches. Outlet water bodies that have water control structures on them that can be opened during excessively wet periods are more effective. Temporary drainage to facilitate repairs on dams, roadways and embankments are another common practice with largely unknown consequences. However, introduction of invasive species is one possible result of drainage (Zedler 2000). Post-drainage subsidence of organic soil layers is common; the lowering of the surface through drying and oxidation can proceed at 1 to 2 cm/year until a stable level is reached (Leifeld et al. 2011). Currently, wetlands are protected by law and manipulations of wetlands are by permit-only. Past drainage practices have left a legacy of land-use mistakes and damaged ecosystems. The value of the ecosystem services of Poor Fens and wetlands in general (flood water retention, surface water purification, habitat for plants and animals) mostly outstrips their development value (Millennium Ecosystem Assessment 2005).
Restoration pathway 2
State 2 to 1
Natural drainage: Drainage can also occur under natural conditions. Beaver dams deteriorate and eventually wash-out. Streams, over time, may cut deeper channels and thereby drain larger areas. Drought may also cause wetland drainage. Following these events, natural short-term ponding patterns may re-emerge. Subsequent to natural drainage, invasive species such as exotic varieties of reed canary grass (Phalaris arundinacea) and Phragmites australis (common reed) as well as purple loosestrife (Lythrum salicaria) may recolonize the site. In the case of man-made impoundments, manipulating water levels to mimic natural drainage patterns presents technological and possibly legal challenges.
Transition 2.A
State 2 to 3
This transition from a Ponded State to a Drained Poor Fen phase is often for the purpose of cranberry production. Ponds in Poor Fens, typically upstream from cranberry bogs, are drained to supply water for cranberry harvest. This water is also used for frost protection during the growing season, flooding the bog over-winter, or weed control. These ponds are not designed or managed for wildlife habitat.
Restoration pathway 3.A
State 3 to 1
Restored wetland hydrology: Wetland restoration is becoming more commonplace. Oftentimes restoration involves backfilling ditches and closing outlets, i. e. reducing drainage of the ecosystem. The intention is to recreate the hydrologic conditions that led to a functioning wetland ecosystem. Unfortunately, it is not always easy to restore wetlands to their former state (Zedler 2000). Typically, fewer native species are found in restored wetlands. Also, invasive species are a serious problem in restored wetlands, they are tenacious competitors, respond well to disturbance and lack some of valuable traits of the natives they have supplanted. There is no reason not to try to restore wetland hydrology; the hydrologic benefits alone can justify the effort. However, the technology must improve to fully realize the potential.
Transition 3.A
State 3 to 2
Prolonged inundation: Cranberry production areas may be ponded for extended periods. Sometimes cranberry operations have unused or abandoned bogs, which often remain ponded and are held in reserve. On a larger scale, most of the numerous flowages of the region have water levels that are changed seasonally, alternately ponding and draining some former Poor Fen sites. Some of the 146 dams in 94D are opened in fall and closed in spring, reversing the natural flood patterns. According the WI DNR online environmental assessment of the Rest Lake Dam, this reversal occurs on the Manitowish River floodplain downstream from that particular dam. Impacts to Poor Fens occur mostly upstream, on sites adjacent to the Manitowish chain of lakes. It is probable that some of these Poor Fens sites have artificially altered hydrology; they are frequently ponded deeper than would occur naturally and then drained rapidly.
Additional community tables
Table 17. Community 1.1 forest overstory composition
| Common name | Symbol | Scientific name | Nativity | Height (ft) | Canopy cover (%) | Diameter (in) | Basal area (square ft/acre) |
|---|---|---|---|---|---|---|---|
|
Tree
|
|||||||
| tamarack | LALA | Larix laricina | Native | 10–20 | 0–1 | 1–4 | – |
Table 18. Community 1.1 forest understory composition
| Common name | Symbol | Scientific name | Nativity | Height (ft) | Canopy cover (%) | |
|---|---|---|---|---|---|---|
|
Grass/grass-like (Graminoids)
|
||||||
| upright sedge | CAST8 | Carex stricta | Native | 1–2.5 | 5–20 | |
| hairy sedge | CALA16 | Carex lacustris | Native | 2–4 | 5–15 | |
| woollyfruit sedge | CALA11 | Carex lasiocarpa | Native | 1–2 | 2–10 | |
| creeping sedge | CACH5 | Carex chordorrhiza | Native | 2–3 | 2–10 | |
| Northwest Territory sedge | CAUT | Carex utriculata | Native | 1–3 | 0–10 | |
| bluejoint | CACA4 | Calamagrostis canadensis | Native | 2–3 | 2–10 | |
| brownish sedge | CABR15 | Carex brunnescens | Native | 1–2 | 1–2 | |
| rattlesnake mannagrass | GLCA | Glyceria canadensis | Native | 2–4 | 1–2 | |
| American mannagrass | GLGR | Glyceria grandis | Native | 2–4 | 1–2 | |
| fowl mannagrass | GLST | Glyceria striata | Native | 2–4 | 1–2 | |
| longhair sedge | CACO8 | Carex comosa | Native | 2–3 | 1–2 | |
| fringed sedge | CACR6 | Carex crinita | Native | 2–3 | 1–2 | |
| greater bladder sedge | CAIN12 | Carex intumescens | Native | 2–3 | 1–2 | |
| threeseeded sedge | CATR10 | Carex trisperma | Native | 1–2 | 1–2 | |
| smooth sawgrass | CLMA | Cladium mariscoides | Native | 1–3 | 1–2 | |
| bristlystalked sedge | CALE10 | Carex leptalea | Native | 2–3 | 1–2 | |
| mud sedge | CALI7 | Carex limosa | Native | 1–2 | 1–2 | |
| tussock cottongrass | ERVA4 | Eriophorum vaginatum | Native | 1–2 | 0–1 | |
| fowl bluegrass | POPA2 | Poa palustris | Native | 1–3 | 0–1 | |
| fringed brome | BRCI2 | Bromus ciliatus | Native | 1–3 | 0–1 | |
|
Forb/Herb
|
||||||
| swamp milkweed | ASIN | Asclepias incarnata | Native | 1–3 | 0–5 | |
| nodding beggartick | BICE | Bidens cernua | Native | 0.5–1.5 | 1–5 | |
| giant goldenrod | SOGI | Solidago gigantea | Native | 3–5 | 1–4 | |
| Canada goldenrod | SOCA6 | Solidago canadensis | Native | 2–4 | 1–3 | |
| swamp verbena | VEHA2 | Verbena hastata | Native | 1–2 | 1–2 | |
| rannoch-rush | SCPA2 | Scheuchzeria palustris | Native | 0.5–1.5 | 1–2 | |
| marsh skullcap | SCGA | Scutellaria galericulata | Native | 0.5–2 | 1–2 | |
| bog goldenrod | SOUL | Solidago uliginosa | Native | 2–3 | 1–2 | |
| northern bog aster | SYBO2 | Symphyotrichum boreale | Native | 1–3 | 1–2 | |
| white panicle aster | SYLA6 | Symphyotrichum lanceolatum | Native | 2–4 | 1–2 | |
| purplestem aster | SYPU | Symphyotrichum puniceum | Native | 2–3 | 1–2 | |
| spotted joe pye weed | EUMA9 | Eutrochium maculatum | Native | 2–3 | 1–2 | |
| rough bedstraw | GAAS2 | Galium asprellum | Native | 0.1–0.2 | 1–2 | |
| yellow avens | GEAL3 | Geum aleppicum | Native | 1–2 | 1–2 | |
| American marshpennywort | HYAM | Hydrocotyle americana | Native | 0.1–0.2 | 1–2 | |
| downy willowherb | EPST | Epilobium strictum | Native | 1–3 | 1–2 | |
| purple marshlocks | COPA28 | Comarum palustre | Native | 1–2 | 1–2 | |
| field horsetail | EQAR | Equisetum arvense | Native | 1–1.5 | 1–2 | |
| common boneset | EUPE3 | Eupatorium perfoliatum | Native | 1–2 | 1–2 | |
| flat-top goldentop | EUGR5 | Euthamia graminifolia | Native | 2–4 | 1–2 | |
| white turtlehead | CHGL2 | Chelone glabra | Native | 1–2 | 1–2 | |
| spotted water hemlock | CIMA2 | Cicuta maculata | Native | 2–4 | 1–2 | |
| purpleleaf willowherb | EPCO | Epilobium coloratum | Native | 1–3 | 1–2 | |
| Arapien blazingstar | MEAR5 | Mentzelia argillosa | Native | 1–2 | 0–1 | |
| bulblet-bearing water hemlock | CIBU | Cicuta bulbifera | Native | 1–3 | 0–1 | |
| giant sunflower | HEGI | Helianthus giganteus | Native | 3–6 | 0–1 | |
| closed gentian | GERU | Gentiana rubricaulis | Native | 0.5–1 | 0–1 | |
| scentbottle | PLDI3 | Platanthera dilatata | Native | 1–2 | 0–1 | |
| buckbean | METR3 | Menyanthes trifoliata | Native | 1–1.5 | 0–1 | |
| Allegheny monkeyflower | MIRI | Mimulus ringens | Native | 0.5–2 | 0–1 | |
| great St. Johnswort | HYAS80 | Hypericum ascyron | Native | 2–4 | 0–1 | |
| American water horehound | LYAM | Lycopus americanus | Native | 1–2 | 0–1 | |
| northern bugleweed | LYUN | Lycopus uniflorus | Native | 1–2 | 0–1 | |
| earth loosestrife | LYTE2 | Lysimachia terrestris | Native | 1–2 | 0–1 | |
| dragon's mouth | ARBU | Arethusa bulbosa | Native | 0.5–1 | 0–1 | |
| marsh bellflower | CAAP2 | Campanula aparinoides | Native | 0.5–2 | 0–1 | |
| tuberous grasspink | CATU5 | Calopogon tuberosus | Native | 1–2 | 0–1 | |
| purple meadow-rue | THDA | Thalictrum dasycarpum | Native | 2–4 | 0–1 | |
| moccasin flower | CYAC3 | Cypripedium acaule | Native | 0.5–1 | 0–0.1 | |
| spoonleaf sundew | DRIN3 | Drosera intermedia | Native | 0.1–0.5 | 0–0.1 | |
|
Fern/fern ally
|
||||||
| sensitive fern | ONSE | Onoclea sensibilis | Native | 0.5–2 | 1–4 | |
| eastern marsh fern | THPA | Thelypteris palustris | Native | 2–3.5 | 1–4 | |
| ostrich fern | MAST | Matteuccia struthiopteris | Native | 2–3 | 1–3 | |
| crested woodfern | DRCR4 | Dryopteris cristata | Native | 1–2 | 0.1–2 | |
| bluntlobe grapefern | BOON | Botrychium oneidense | Native | 0–0.5 | 0–0.1 | |
|
Shrub/Subshrub
|
||||||
| speckled alder | ALINR | Alnus incana ssp. rugosa | Native | 3–10 | 2–10 | |
| leatherleaf | CHCA2 | Chamaedaphne calyculata | Native | 1.5–2.5 | 2–10 | |
| white meadowsweet | SPAL2 | Spiraea alba | Native | 2–4 | 1–10 | |
| redosier dogwood | COSE16 | Cornus sericea | Native | 3–6 | 1–5 | |
| meadow willow | SAPE5 | Salix petiolaris | Native | 3–10 | 2–5 | |
| steeplebush | SPTO2 | Spiraea tomentosa | Native | 2–4 | 1–3 | |
| withe-rod | VINUC | Viburnum nudum var. cassinoides | Native | 4–8 | 0–1 | |
| bog birch | BEPU4 | Betula pumila | Native | 3–8 | 0–1 | |
| nannyberry | VILE | Viburnum lentago | Native | 4–8 | 0–1 | |
|
Nonvascular
|
||||||
| sphagnum | SPFI4 | Sphagnum fimbriatum | Native | 0.1–0.2 | 5–20 | |
| sphagnum | SPMA11 | Sphagnum majus | Native | 0.1–0.2 | 1–5 | |
| polytrichum moss | POCO38 | Polytrichum commune | Native | 0.1–0.2 | 1–5 | |
| polytrichum moss | POST70 | Polytrichum strictum | Native | 0.1–0.2 | 1–5 | |
Table 19. Community 1.2 forest understory composition
| Common name | Symbol | Scientific name | Nativity | Height (ft) | Canopy cover (%) | |
|---|---|---|---|---|---|---|
|
Grass/grass-like (Graminoids)
|
||||||
| woollyfruit sedge | CALA11 | Carex lasiocarpa | Native | 1–1.5 | 2–10 | |
| creeping sedge | CACH5 | Carex chordorrhiza | Native | 1–2 | 5–10 | |
| hairy sedge | CALA16 | Carex lacustris | Native | 2–3 | 5–10 | |
| mud sedge | CALI7 | Carex limosa | Native | 1–1.5 | 1–5 | |
| fewseed sedge | CAOL3 | Carex oligosperma | Native | 1–1.5 | 1–5 | |
| bluejoint | CACA4 | Calamagrostis canadensis | Native | 2–3 | 1–5 | |
| greater bladder sedge | CAIN12 | Carex intumescens | Native | 1–2 | 1–5 | |
| bristlystalked sedge | CALE10 | Carex leptalea | Native | 1–2 | 1–5 | |
| threeseeded sedge | CATR10 | Carex trisperma | Native | 1–1.5 | 1–5 | |
| Northwest Territory sedge | CAUT | Carex utriculata | Native | 1–1.5 | 1–5 | |
| brownish sedge | CABR15 | Carex brunnescens | Native | 0.8–1.2 | 2–5 | |
| common rush | JUEF | Juncus effusus | Native | 2–3 | 1–2 | |
| seaside arrowgrass | TRMA20 | Triglochin maritima | Native | 1–2 | 1–2 | |
| white beaksedge | RHAL3 | Rhynchospora alba | Native | 1–2 | 1–2 | |
| tall cottongrass | ERAN6 | Eriophorum angustifolium | Native | 1–1.5 | 1–2 | |
| tussock cottongrass | ERVA4 | Eriophorum vaginatum | Native | 1–1.5 | 1–2 | |
| three-way sedge | DUAR3 | Dulichium arundinaceum | Native | 1–2 | 0–1 | |
|
Forb/Herb
|
||||||
| Canadian rush | JUCA3 | Juncus canadensis | Native | 2–3 | 1–2 | |
| tumblegrass | SCPA | Schedonnardus paniculatus | Native | 0.6–1.2 | 1–2 | |
| dwarf violet iris | IRVE | Iris verna | Native | 2–3 | 1–2 | |
| threeleaf false lily of the valley | MATR4 | Maianthemum trifolium | Native | 0.2–0.4 | 1–2 | |
| scentbottle | PLDI3 | Platanthera dilatata | Native | 1–2 | 0–1 | |
| snakemouth orchid | POOP | Pogonia ophioglossoides | Native | 0.6–1 | 0–1 | |
| purple pitcherplant | SAPU4 | Sarracenia purpurea | Native | 0.8–1.2 | 0–1 | |
| spoonleaf sundew | DRIN3 | Drosera intermedia | Native | 0.2–0.6 | 0–1 | |
| roundleaf sundew | DRRO | Drosera rotundifolia | Native | 0.2–0.6 | 0–1 | |
| northern bog bedstraw | GALA2 | Galium labradoricum | Native | 0.2–0.4 | 0–1 | |
|
Fern/fern ally
|
||||||
| eastern marsh fern | THPA | Thelypteris palustris | Native | 1–2.5 | 1–2 | |
| sensitive fern | ONSE | Onoclea sensibilis | Native | 0.5–1 | 1–2 | |
|
Shrub/Subshrub
|
||||||
| leatherleaf | CHCA2 | Chamaedaphne calyculata | Native | 1–2.5 | 5–15 | |
| meadow willow | SAPE5 | Salix petiolaris | Native | 3–6 | 0–2 | |
| speckled alder | ALINR | Alnus incana ssp. rugosa | Native | 3–5 | 1–2 | |
| sweetgale | MYGA | Myrica gale | Native | 2–4 | 0–1 | |
| bog birch | BEPU4 | Betula pumila | – | 3–5 | 0–1 | |
|
Vine/Liana
|
||||||
| small cranberry | VAOX | Vaccinium oxycoccos | Native | – | 0–1 | |
|
Nonvascular
|
||||||
| sphagnum | SPFI4 | Sphagnum fimbriatum | Native | 0.1–0.2 | 10–20 | |
| sphagnum | SPMA11 | Sphagnum majus | Native | 0.1–0.2 | 10–20 | |
| Magellan's sphagnum | SPMA70 | Sphagnum magellanicum | Native | 0.1–0.2 | 5–10 | |
| polytrichum moss | POCO38 | Polytrichum commune | Native | 0.1–0.2 | 5–10 | |
| polytrichum moss | POST70 | Polytrichum strictum | Native | 0.1–0.2 | 2–10 | |
| sphagnum | SPCA70 | Sphagnum capillifolium | Native | 0.1–0.2 | 5–10 | |
| toothed sphagnum | SPCU4 | Sphagnum cuspidatum | Native | 0.1–0.2 | 5–10 | |
| sphagnum | SPSU9 | Sphagnum subsecundum | Native | 0.1–0.2 | 5–10 | |
| club spikemoss | SESE | Selaginella selaginoides | Native | 0.2–0.4 | 0–1 | |
| papillose sphagnum | SPPA71 | Sphagnum papillosum | Native | 5–10 | 0.1–0.2 | |
Table 20. Community 2.1 forest understory composition
| Common name | Symbol | Scientific name | Nativity | Height (ft) | Canopy cover (%) | |
|---|---|---|---|---|---|---|
|
Grass/grass-like (Graminoids)
|
||||||
| broadleaf cattail | TYLA | Typha latifolia | Native | 3–5 | 2–10 | |
| woolgrass | SCCY | Scirpus cyperinus | Native | 1–3 | 1–5 | |
| water sedge | CAAQ | Carex aquatilis | Native | 0.5–1.5 | 1–2 | |
| Canadian rush | JUCA3 | Juncus canadensis | Native | 1–3 | 1–2 | |
| common rush | JUEF | Juncus effusus | Native | 1–3 | 1–2 | |
| northern wildrice | ZIPAI | Zizania palustris var. interior | Native | 2–4 | 0–2 | |
| green bulrush | SCAT2 | Scirpus atrovirens | Native | 1–3 | 1–2 | |
| white beaksedge | RHAL3 | Rhynchospora alba | Native | 1–2 | 0–1 | |
| panicled bulrush | SCMI2 | Scirpus microcarpus | Native | 1–3 | 0–1 | |
|
Forb/Herb
|
||||||
| horned bladderwort | UTCO | Utricularia cornuta | Native | 0.2–1 | 1–3 | |
| flatleaf bladderwort | UTIN2 | Utricularia intermedia | Native | 0.2–0.8 | 1–2 | |
| broadleaf arrowhead | SALA2 | Sagittaria latifolia | Native | 0.5–3 | 1–2 | |
| watershield | BRSC | Brasenia schreberi | Native | 2–6 | 0–2 | |
| water arum | CAPA | Calla palustris | Native | 1–3 | 1–2 | |
| harlequin blueflag | IRVE2 | Iris versicolor | Native | 2–3 | 1–2 | |
| pickerelweed | POCO14 | Pontederia cordata | Native | 1–2 | 1–2 | |
| jewelweed | IMCA | Impatiens capensis | Native | 0.5–2 | 1–2 | |
| Shreve's iris | IRVIS | Iris virginica var. shrevei | Native | 2–3 | 0–1 | |
| greater water dock | RUORB | Rumex orbiculatus var. borealis | Native | 2–4 | 0–1 | |
| lavender bladderwort | UTRE | Utricularia resupinata | Native | 0.1–0.3 | 0–1 | |
| eastern purple bladderwort | UTPU | Utricularia purpurea | Native | 0.1–0.2 | 0–1 | |
Table 21. Community 3.1 forest overstory composition
| Common name | Symbol | Scientific name | Nativity | Height (ft) | Canopy cover (%) | Diameter (in) | Basal area (square ft/acre) |
|---|---|---|---|---|---|---|---|
|
Tree
|
|||||||
| quaking aspen | POTR5 | Populus tremuloides | Native | 3–30 | 0–5 | 1–6 | – |
Table 22. Community 3.1 forest understory composition
| Common name | Symbol | Scientific name | Nativity | Height (ft) | Canopy cover (%) | |
|---|---|---|---|---|---|---|
|
Grass/grass-like (Graminoids)
|
||||||
| bluejoint | CACA4 | Calamagrostis canadensis | Native | 2–3.5 | 10–20 | |
| reed canarygrass | PHAR3 | Phalaris arundinacea | Introduced | 2–3.5 | 10–20 | |
| common reed | PHAU7 | Phragmites australis | Introduced | 4–8 | 0–10 | |
|
Forb/Herb
|
||||||
| Canada goldenrod | SOAL6 | Solidago altissima | Native | 3–4 | 5–15 | |
| common milkweed | ASSY | Asclepias syriaca | Native | 2–3.5 | 5–10 | |
| purple loosestrife | LYSA2 | Lythrum salicaria | Introduced | 2–3 | 0–10 | |
| cowparsnip | HERAC | Heracleum | Native | 3–5 | 5–10 | |
| yellow avens | GEAL3 | Geum aleppicum | Native | 1.5–3 | 1–5 | |
| tall buttercup | RAAC3 | Ranunculus acris | Introduced | 1–3 | 1–5 | |
|
Fern/fern ally
|
||||||
| field horsetail | EQAR | Equisetum arvense | Native | 1–2 | 1–5 | |
|
Shrub/Subshrub
|
||||||
| American red raspberry | RUID | Rubus idaeus | Native | 2–3 | 5–15 | |
| willow | SALIX | Salix | Native | 4–8 | 5–10 | |
Interpretations
Supporting information
Inventory data references
Reference Site 1: Powell Marsh State Wildlife Area, Vilas County, Wisconsin.
Latitude: 46.090506 Longitude: -89.887413
Legal Description: 2400 feet north and 300 feet east of the southwest corner of Section 28, T 42 N - R 5 W.
Site Properties: This site is located within a large peatland ecological site complex; site about 20% ponded (in hollows); about 1% slope to the south.
Soil Properties: Thin peat moss layer at surface - pH 4.9; Muck subsurface layer to 49 inches-pH 4.7; Stratified loamy fine sand and fine sandy loam to >150 inches-pH 4.8
Dominant Species: Carex lacustris, Calamagrostis canadensis, Alnus incana, Betula pumila, Chamaedaphne calyculata; indicates Sedges-Shrubs phase
Reference Site 2: Thunder Lake State Wildlife Area, Oneida County, Wisconsin.
Latitude: 45.817352 Longitude: -89.225830
Legal Description: SW1/4, SW1/4, SE1/4 of Section 34, T 39 N – R 10 W.
Site Properties: site is located near Rice Lake, part of large peatland of mostly Poor Fen sites interspersed with man-made ponds; saturated to surface; about 20% of site is ponded; old ditch and road network across the site; about 1% slope to west, drains to Rice Lake
Soil Properties: Mucky Peat and Peat surface layer-pH 4.5; Muck subsurface layer to 24 inches-pH 4.7; Sand substratum to > 150 inches-pH 5.2
Dominant Species: Carex lacustris, Calamagrostis canadensis, Salix petiolaris, Spirea alba; indicates Sedges-Shrubs phase
Reference Site 3: Dragonfly Pond area, Treehaven Education and Conference Center, Lincoln County, Wisconsin.
Latitude: 45.817352 Longitude: -89.547664
Legal Description: 1600 feet south and 270 feet east of the northwest corner of Section 19, T 35 N – R 8 W.
Site Properties: ponded throughout 2014 due to second wettest year on record; stand of tamarack on north end (Mucky Peat Bog ecological site); site drains to south into Dragonfly Pond; slope <1%
Soil Properties: Mucky peat surface layer--pH 4.6; Muck subsurface layer to > 51 inches (130 cm)--pH 4.7
Dominant Species: Carex lasiocarpa, Eriophorum spp., Sphagnum spp., Chamaedaphne calyculata; indicates Sedges-Sphagnum phase
Other references
Attig JW. 1985 Pleistocene geology of Vilas County, Wisconsin. Wis. Geol. and Nat. Hist. Surv. Information Circular 50. 38 pp.
Black MR., Judziewicz EJ. 2009. Wildflowers of Wisconsin and the Great Lakes Region: a comprehensive field guide. 2nd ed. Univ. Wisc. Press 275pp.
Bertness MD, Hacker SD. 1994. Physical stress and positive associations among marsh plants. American Naturalist 144: 363-372.
Boelter DH, Verry ES. 1977. Peatland and water in the northern Lake States. General Tech. Report NC-31, North Cent. Forest Exp. Station, USDA-Forest Service. 22 pp.
Brinson M. 1993. A Hydrogeomorphic classification of wetlands. US Army COE. 101 pp.
Chapin CT, Bridgham SD, Pastor J. 2004. pH and nutrient effects on above-ground net primary productivity in a Minnesota, USA bog and fen. Wetlands 24(1):186-201.
Cohen JG, Kost MA. 2008. Natural community abstract for poor fen. Michigan Natural Features Inventory, Lansing, MI. 17 pp.
Crum H. 1988. A Focus on Peatlands and Peat Mosses. Univ. Mich. Press. 306 pp.
Curtis JT. 1971. The Vegetation of Wisconsin: an ordination of plant communities. Univ. Wisc. Press. 657 pp.
ECOMAP. 1993. National hierarchical framework of ecological units. USDA Forest Service, Washington, D.C.
Epstein E, Smith W, Dobberpuhl J, Galvin A. 1999. Biotic inventory and analysis of the Northern Highland-American Legion State Forest. Bureau of Endangered Resources, Wisconsin Department of Natural Resources. 263pp.
Faber-Langedoen D, editor. 2001. Plant communities of the Midwest: Classification in an ecological context. Association for Biodiversity Information, Arlington, VA. 61 pp. + appendix (705 pp.).
Federal Geographic Data Committee. 2013. Classification of wetlands and deep water habitats of the United States. FGDC-STD-004-2013. Second Edition. Wetlands Subcommittee, Federal Geographic Data Committee and U.S. Fish and Wildlife Service, Washington, DC.
Frolking S, Roulet NT, Moore TR, Richard PJH, Lavoie M, Muller SD. 2001. Modelling northern peatland decomposition and peat accumulation. Ecosystems 4:479-498.
Heinselman ML. 1963. Forest sites, bog processes, and peatland types in the glacial lake Agassiz region, Minnesota. Ecol. Monographs 33:327-374.
Hipp AL. 2008. Field Guide to Wisconsin Sedges: An introduction to the genus Carex (Cyperaceae). Univ. Wisc. Press. 265pp.
Hribljan JA. 2012. The effect of long-term water table manipulations on vegetation, pore water, substrate quality, and carbon cycling in a northern poor fen peatland. PhD dissertation Mich. Tech. Univ.
Judziewicz EJ, Freckman RW, Clark LG, Black MR. 2014. Field Guide to Wisconsin Grasses. Univ. Wisc. Press. 346pp.
Kotar J, Burger TL. 2009. Habitat Type Classification for Wetland Forests, Region 3 (prelim. ver.) University of Wisconsin-Madison, Dept. of Forest and Wildlife Ecology. 43 pp.
Kotar J, Kovach JA, Burger TL. 2002. A Guide to Forest Communities and Habitat Types of Northern Wisconsin. 2nd ed. University of Wisconsin-Madison, Dept. of Forest Ecology and Management.
Kowalski KP, Wilcox DA. 2003. Differences in sedge fen vegetation upstream and downstream from a managed impoundment. American Midland Naturalist 150(2):199-220.
Kozlowski TT, Pallardy SG. 2002. Acclimation and adaptive responses of woody plants to environmental stresses. The Botanical Review 68(2): 270-334.
Leifeld J, Muller M, Fuhrer J. 2011. Peatland subsidence and carbon loss from drained temperate fens. Soil Use and Management 27(2):170-176.
Millennium Ecosystem Assessment. 2005. ECOSYSTEMS AND HUMAN WELL-BEING: WETLANDS AND WATER Synthesis. World Resources Institute, Washington, DC
Mitchell SJ. 2013. Wind as a natural disturbance in forests; a synthesis. Forestry 86:147-157.
Natural Resources Conservation Service. 2008. Hydrogeomorphic Wetland Classification System: An overview and modification to better meet the needs of the Natural Resources Conservation Service. Technical Note No. 190–8–76.
Novitzki R. 1982. Hydrology of Wisconsin Wetlands. Info. Circ. 40 Wis. Geol. and Nat. Hist. Surv. 22 pp.
Pielou EC. 1991. After the Ice Age: the return of life to glaciated North America. Univ. Chicago Press, Chicago, IL. 366 pp.
Rydin H, Jeglum J. 2013. The Biology of Peatlands, 2nd ed. Oxford Univ. Press 382 pp.
Scheffer, RA, Van Logtestijn, RS, Verhoeven, JTA. 2001. Decomposition of Carex and Sphagnum litter in two mesotrophic fens differing in dominant plant species. Oikos 92: 44–54.
Venterink HO, van der Vliet RE, Wassen MJ. 2001. Nutrient limitation along a productivity gradient in wet meadows. Plant and Soil 234:171-179.
Weltzin JF, Bridgham SD, Pastor J, Chen J, Harth C. 2002. Potential effects of warming and drying on peatland plant community composition. Global Change Biology 9:141-151.
Weltzin JF, Pastor J, Harth C, Bridgham SD, Updegraff K, Chapin CT. 2000. Response of bog and fen plant communities to warming and water-table manipulations. Ecology 81(12):3464-3478
.
Wisconsin Department of Natural Resources (DNR). 2014. The ecological landscapes of Wisconsin: an assessment of ecological resources and a guide to planning sustainable management. Chapter 14, Northern Highland Ecological Landscape. Wisconsin Department of Natural Resources, PUB-SS-1131P 2014, Madison. 84 pp.
Wisconsin Initiative on Climate Change Impacts (WICCI) 2011. Wisconsin’s Changing Climate: Impacts and Adaptations. Nelson Institute for Environmental Studies, University of Wisconsin-Madison & the Wisconsin Department of Natural Resources, Madison, Wisconsin.
Zedler JB. 2000. Progress in wetland restoration ecology. Trends in Ecology and Evolution 15(10):402-407.
Zobel RW. 1992. Soil environment constraints to root growth. Adv. Soil Science 19:27-51.
Zweig CL, Kitchens WM. 2009. Multi-state succession in wetlands: a novel use of state and transition models. Ecology 90(7):1900-1909.
Contributors
Mark Krupinski
Approval
Suzanne Mayne-Kinney, 3/29/2024
Rangeland health reference sheet
Interpreting Indicators of Rangeland Health is a qualitative assessment protocol used to determine ecosystem condition based on benchmark characteristics described in the Reference Sheet. A suite of 17 (or more) indicators are typically considered in an assessment. The ecological site(s) representative of an assessment location must be known prior to applying the protocol and must be verified based on soils and climate. Current plant community cannot be used to identify the ecological site.
| Author(s)/participant(s) | |
|---|---|
| Contact for lead author | |
| Date | 03/29/2024 |
| Approved by | Suzanne Mayne-Kinney |
| Approval date | |
| Composition (Indicators 10 and 12) based on | Annual Production |
Indicators
-
Number and extent of rills:
-
Presence of water flow patterns:
-
Number and height of erosional pedestals or terracettes:
-
Bare ground from Ecological Site Description or other studies (rock, litter, lichen, moss, plant canopy are not bare ground):
-
Number of gullies and erosion associated with gullies:
-
Extent of wind scoured, blowouts and/or depositional areas:
-
Amount of litter movement (describe size and distance expected to travel):
-
Soil surface (top few mm) resistance to erosion (stability values are averages - most sites will show a range of values):
-
Soil surface structure and SOM content (include type of structure and A-horizon color and thickness):
-
Effect of community phase composition (relative proportion of different functional groups) and spatial distribution on infiltration and runoff:
-
Presence and thickness of compaction layer (usually none; describe soil profile features which may be mistaken for compaction on this site):
-
Functional/Structural Groups (list in order of descending dominance by above-ground annual-production or live foliar cover using symbols: >>, >, = to indicate much greater than, greater than, and equal to):
Dominant:
Sub-dominant:
Other:
Additional:
-
Amount of plant mortality and decadence (include which functional groups are expected to show mortality or decadence):
-
Average percent litter cover (%) and depth ( in):
-
Expected annual annual-production (this is TOTAL above-ground annual-production, not just forage annual-production):
-
Potential invasive (including noxious) species (native and non-native). List species which BOTH characterize degraded states and have the potential to become a dominant or co-dominant species on the ecological site if their future establishment and growth is not actively controlled by management interventions. Species that become dominant for only one to several years (e.g., short-term response to drought or wildfire) are not invasive plants. Note that unlike other indicators, we are describing what is NOT expected in the reference state for the ecological site:
-
Perennial plant reproductive capability:
Print Options
Sections
Font
Other
The Ecosystem Dynamics Interpretive Tool is an information system framework developed by the USDA-ARS Jornada Experimental Range, USDA Natural Resources Conservation Service, and New Mexico State University.
Click on box and path labels to scroll to the respective text.