

Natural Resources
Conservation Service
Ecological site R144AY001CT
Tidal Salt Low Marsh mesic very frequently flooded
Last updated: 10/04/2024
Accessed: 02/28/2026
General information
Approved. An approved ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model, enough information to identify the ecological site, and full documentation for all ecosystem states contained in the state and transition model.
Figure 1. Mapped extent
Areas shown in blue indicate the maximum mapped extent of this ecological site. Other ecological sites likely occur within the highlighted areas. It is also possible for this ecological site to occur outside of highlighted areas if detailed soil survey has not been completed or recently updated.
MLRA notes
Major Land Resource Area (MLRA): 144A–New England and Eastern New York Upland, Southern Part
MLRA 145, Connecticut Valley is based on the concept that glacial Lake Hitchock left finer sediments in the form of varved clays, silts, and fine sands in the middle of a larger and coarser-textured post-glacial environment. The upland areas are mainly comprised of glacial deposits (till and outwash) and/or loess overlying glacial lake sediments, with a mix of recent alluvium and organic residuum in the lower-lying areas directly surrounding the Connecticut River.
The tidal marsh ecological sites are located in the southern section of the MLRA, near the mouth of the Connecticut River along the Long Island Sound coast.
MLRA 144A, the New England and Eastern New York Upland, Southern Part is in the New England Upland section of the New England Province of the Appalachian Highlands Division. The area is nearly level to sloping lowlands on the edges of the valley. North to south running trap rock ridges break up the lowlands with hilly, steep areas. Elevation ranges from sea level to 330 feet (100 meters) in the lowlands and from 650 feet to 1,000 feet (200- 305 meters) on ridges.
The tidal marsh ecological sites are located in the south and eastern sections of the MLRA, near the Atlantic Ocean and Long Island Sound coasts.
MLRA 149B, Long Island-Cape Cod Coastal Lowland is in the Embayed section of the Coastal Plain Province of the Atlantic Plain Division (Fenneman & Johnson, 1946). It is part of the partially submerged coastal plain of New England. It is mostly an area of nearly level to rolling plains, but it has some steeper hills (glacial moraines). Ridges border the lower plains. Elevation generally ranges from sea level to 80 feet (0 to 25 meters), but it is as much as 410 feet (125 meters) in a few areas.
The tidal marsh ecological sites are located throughout the MLRA, near the Atlantic Ocean and Long Island Sound coasts.
Classification relationships
Phytotaxomomically
CT: Salt Marshes - Smooth Cordgrass (Spartina alterniflora) community; Slender glasswort – Smooth cordgrass (Salicornia europea - Spartina alterniflora) community; Virginia glasswort (Salicornia virginica) tidally flooded vegetation (Metzler, and Barrett 2006).
MA: Estaurine Intertidal: Salt Marsh (Swain and Kearsley 2012)
ME: Tidal Marsh Estuary Ecosystem includes: Mixed Saltmarsh (Gawler and Cutko 2010).
NJ: Salt marsh complex. (Breden 1989)
NH: Low Salt Marsh (Sperduto and Nichols 2012)
NY: Low salt marsh (Edinger et al. 2002)
RI: Salt Marsh System, includes Low salt marsh (Enser et al. 2011).
NatureServe:
CEGL004308 Salicornia (virginica, bigelovii, maritima) - Spartina alterniflora Herbaceous Vegetation
CEGL004192 Spartina alterniflora / (Ascophyllum nodosum) Acadian/Virginian Zone Herbaceous Vegetation
2. Cartographically:
US Forest Service ECOMAP in Keys, J. A. et al. 1995. Ecological Units of
the Eastern United States - First Approximation (Map 1:3,500,00 + Booklet) US Department of Agriculture Forest Service, Atlanta, GA
221A-Lower New England Section
221Aa Boston Basin Subsection
221Ab Cape Cod Coastal Lowland and Islands Subsection
221Ac Narragansett-Bristol Lowland and Islands Subsection
221Ad Southern New England Coastal Lowland Subsection
221Ai Gulf of Maine Coastal Plain Subsection
221An Long Island Coastal Lowland and Moraine Subsection
Ecological site concept
Salt marshes are coastal wetlands that are alternately flooded and drained by salt water borne by oceanic tides. Salt marshes typically develop in sheltered environments in different geomorphic settings, typically coves, bays, behind barrier beaches, drowned valleys, and at the confluence of major rivers, where marshy peat and sediment can accumulate. As coastal landforms, tidal marshes are low in elevation and generally flat, although they can exhibit changes in microrelief. The climate depends upon latitude, but evidently with a strong maritime influence, producing cooler summers and warmer winters. Tidal marsh vegetation is poorly developed but highly productive. Few plant species can physiologically tolerate the high salinities, but those that do can flourish. The predominant vegetation form in the regularly flooded zone is the tidal salt “low marsh” appearing as different vegetation-forms reflecting changes in density and composition, Several different states occur where the vegetation and habitat conditions change dramatically, including a dieback/ or collapsed state and a tidally restricted state, impounded state, and filled state. In addition are 4 transition crossovers to other ecological sites: Tidal Brackish Wetland ecological site, Tidal Salt High Marsh ecological site, Tidal Salt Flats ecological site, and various Filled Nontidal ecological sites.
Similar sites
| R144AY002CT |
Tidal Salt High Marsh mesic very frequently flooded |
|---|
Table 1. Dominant plant species
| Tree |
Not specified |
|---|---|
| Shrub |
Not specified |
| Herbaceous |
(1) Spartina alterniflora |
Physiographic features
This ecological site occupies the lowest portions of the terrestrial landscape bordering the coast as a narrow intertidal zone primarily composed of organic matter. Local relief is 0 to 4 feet (0 to 1.2 meters) with slopes ranging from 0 to 2 percent (mean slope of about 1 percent).
A water table is found within a few centimeters of the soil surface due to tidal flooding which occurs about twice daily, during the high tides. Runoff or groundwater discharge may be recieved at this physiographic site from surrounding uplands
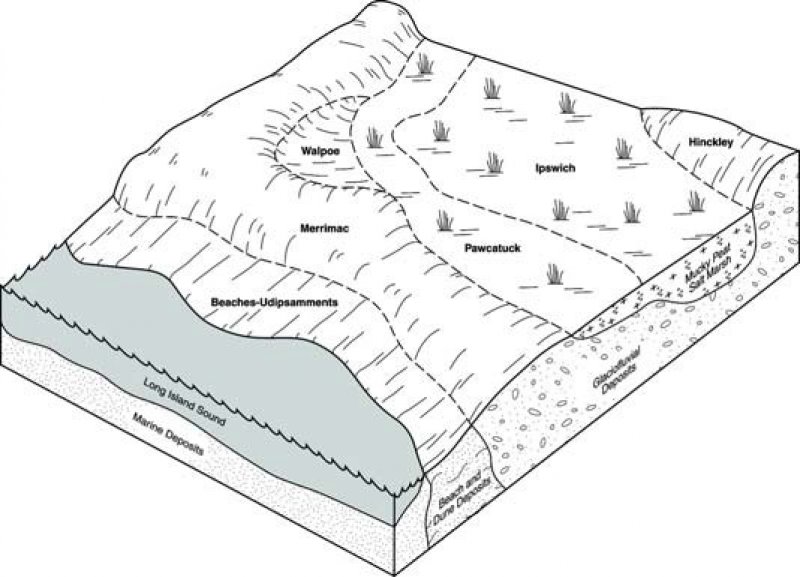
Figure 2. Salt Marsh Block Diagram
Table 2. Representative physiographic features
| Landforms |
(1)
Coastal plain
> Tidal marsh
(2) Back-barrier flat |
|---|---|
| Runoff class | Negligible |
| Flooding duration | Extremely brief (0.1 to 4 hours) to very brief (4 to 48 hours) |
| Flooding frequency | Very frequent |
| Ponding frequency | None |
| Elevation | 9 ft |
| Slope | 2% |
| Water table depth |
Not specified |
| Aspect | Aspect is not a significant factor |
Climatic features
The Koppen-Geiger climate classification of the area in which this MLRA occurs varies between Dfb (Warm-summer humid continental) in the North, and Dfa (Hot-summer humid continental) in the southern portion of the MLRA. Precipitation is usually uniformly distributed throughout the year. Near the coast, however, it is slightly lower in summer. Precipitation is slightly higher in spring and fall in inland areas. Rainfall occurs as high-intensity, convective thunderstorms during the summer. Thunderstorms occur on about 20 days each year, and most occur in July. Monthly precipitation in the Northeast is fairly uniform throughout the year. Occasional drought is a normal, recurrent feature of virtually every climate in the United States. However, even with a temperate moist climate, normal fluctuations in regional weather patterns can lead to periods of dry weather. During the winter, most of the precipitation occurs as moderate-intensity storms (northeasters) that produce large amounts of rain or snow. The freeze-free period increases in length to the south.
The Atlantic hurricane season runs from June 1st to November 30th. The estimated return period for hurricanes passing within 50 nautical miles of this area of coastal New York and New England ranges 13-43 years with an average of 22.3 years. The return period for major (i.e. Category 3 or greater) hurricanes ranges 52-180 years with an average of 88.0 years. From 1950-2011, these coastal counties have had four Category III hurricane strikes recorded in 1954 (2), 1960 and 1985. They have had one Category II hurricane strike in 1991 and one Category I hurricane strike in 1976 (NOAA NWS 2013).
Future projections indicate that greenhouse warming will cause the globally averaged intensity of tropical cyclones to shift towards stronger storms, with intensity increases of 2–11% by 2100 (Knutson et al 2010).
Table 3. Representative climatic features
| Frost-free period (characteristic range) | 116-160 days |
|---|---|
| Freeze-free period (characteristic range) | 144-203 days |
| Precipitation total (characteristic range) | 46-50 in |
| Frost-free period (actual range) | 115-176 days |
| Freeze-free period (actual range) | 130-212 days |
| Precipitation total (actual range) | 44-51 in |
| Frost-free period (average) | 142 days |
| Freeze-free period (average) | 172 days |
| Precipitation total (average) | 48 in |
Figure 3. Monthly precipitation range
Figure 4. Monthly minimum temperature range
Figure 5. Monthly maximum temperature range
Figure 6. Monthly average minimum and maximum temperature
Figure 7. Annual precipitation pattern
Figure 8. Annual average temperature pattern
Climate stations used
-
(1) TETERBORO AP [USW00094741], Moonachie, NJ
-
(2) BRIDGEPORT SIKORSKY MEM AP [USW00094702], Stratford, CT
-
(3) WESTERLY STATE AP [USW00014794], Westerly, RI
-
(4) PLYMOUTH MUNI AP [USW00054769], Carver, MA
-
(5) GREENLAND [USC00273626], Greenland, NH
Influencing water features
Very poorly drained
Water is removed from the soil so slowly that free water remains at or very near the surface during much of the growing season. Internal free water occurrence is very shallow and persistent or permanent. Unless the soil is artificially drained, most mesophytic crops cannot be grown. The soils are commonly level or depressed and frequently ponded. In areas where rainfall is high or nearly continuous, slope gradients may be greater.
Water is one of the limiting factors in this system. Saturated conditions slow microbial break down and allow organic matter to accumulate over mineral material. Tidal flooding (twice daily during high tide) keeps halinity values and pH high in the system, which controls the vegetation types. As you move up the river or further inland from the shore, these effects diminish.
Wetland description
National Wetland Classification (Cowardin et al., 1979):
System: Estuarine
Subsystem: Intertidal
Class: Unconsolidated Bottom, Aquatic Bed, Emergent
Subclass: Cobble-Gravel, Sand, Mud, Algal, Rooted/Floating Vascular, Organic, Persistent/Non-persistent, Phragmites australis
Water Regime: Regularly Flooded
Soil features
This ecological site is represented by soils in the Histosols and Entisols soil orders. Major soil series for this ecological site are Ipswich and Westbrook with some areas of Sandyhook and Pawcatuck. Depth of organic material is variable.
These soils have a mesic soil temperature regime, an aquic soil moisture regime, and mixed mineralogy (Soil Survey Staff, Official Series Descriptions, available online). They have histic epipedons that are shallow to deep to a mineral material (alluvium, glacial till, outwash, or marine deposits) and generally have a sandy or sandy skeletal or a coarse-loamy particle size class when applicable. There does not seem to be a correlation between depth to mineral material and vegetative community type.
A seasonal high water table (SHWT) is at the surface due to tidal flooding. The soil series associated with this ecological site are interpreted to be very poorly drained according to Connecticut drainage class standards. Saturated hydraulic conductivity in the soil material is moderately high to very high (Soil Survey Staff, 2013).
Soils associated with this ecological site are formed in organic material deposited over mineral till, outwash, alluvium, or marine deposits. The organic material is derived mainly from native herbacious tidal marsh species, but has been found to be woody with depth as a relict of post-glacial sea level rise. Though most of the material is composed of organic material which generates acids as it breaks down, the salts and minerals deposited by the ocean keep the pH and EC values values high. These unique chemical properties along with sustained periods of saturation are what allow the Spartina species and other salt marsh vegetation to out-compete other species and give the marshes their distinct community banding.

Figure 9. Tidal Marsh Soil Profile
Table 4. Representative soil features
| Parent material |
(1)
Herbaceous organic material
(2) Marine deposits |
|---|---|
| Surface texture |
(1) Mucky |
| Family particle size |
(1) Sandy (2) Loamy (3) Sandy or sandy-skeletal |
| Drainage class | Very poorly drained to poorly drained |
| Permeability class | Slow |
| Depth to restrictive layer | 72 in |
| Surface fragment cover <=3" | Not specified |
| Surface fragment cover >3" | Not specified |
| Available water capacity (Depth not specified) |
4 – 18 in |
| Electrical conductivity (0-40in) |
1 – 112 mmhos/cm |
| Soil reaction (1:1 water) (0-40in) |
4 – 8.4 |
| Subsurface fragment volume <=3" (Depth not specified) |
Not specified |
| Subsurface fragment volume >3" (Depth not specified) |
Not specified |
Ecological dynamics
Preface: Different classes of tidal wetlands - changes in salinity and tidal position
Salinity.
Situated between the land and the sea, tidal wetlands are defined in terms of salinity levels and by the vertical extent of the tides. Tidal wetlands include all wetlands subject to the influence of the tides yet vary in salinity, ranging from undiluted seawater to freshwater derived from inland sources such as rivers, runoff, and groundwater seeps. These broad variations in salinity create an intergrading sequence of tidal wetlands ranging from: (1) salt tidal marshes, to (2) brackish tidal wetlands, to (3) freshwater tidal wetlands. The relationship between salinity and wetland class can be approximated by Cowardin et al. (Cowardin et al. 1979, Theve 2013). (In practice, the term “halinity” is sometimes used in place of salinity to denote ocean salts dominated by halide, NaCl. The units may be expressed in parts-per-thousand [ppt] or virtually equivalent Practical Salinity Units [PSU] (UNESCO 1981) or commonly expressed as deciSiemans-per-meter [dSm-1]. An approximate conservative conversion is 1 dSm-1 = 0.640 ppt at 25 °C.) Salt marshes typically exhibit salinity levels greater than 18 ppt (28.1 dSm-1), considered polyhaline, 18 - 30 ppt (28.1- 46.9 dSm-1) and levels greater than 30 ppt (46.9 dSm-1), especially in situations where surface evaporation can concentrate salts. The salinity levels of brackish wetlands are more dilute ranging from 0.5 - 18 ppt (0.8 – 28.1 dSm-1), considered oligohaline, 0.5 – 5 ppt (0.8 – 7.8 dSm-1), to mesohaline, 5 – 18 ppt (7.8 – 28.1 dSm-1). Freshwater tidal wetlands exhibit salinity levels of 0.5 ppt (0.8 dSm-1) or less. Salt and brackish tidal marshes are considered estuarine habitats, and are directly influenced by tidal flooding. Salt marshes are found in sheltered, depositional areas located in coves, embayments, and behind barrier beaches. Brackish marshes exist either along landward seeps or drainageways of rivers and streams where fresh and saline waters mix. Freshwater tidal wetlands, while still physically affected by tidal forces, are beyond the reach of the salt front. Lacking salinity, freshwater tidal habitats are sometimes considered riverine habitats (Cowardin et al. 1979), yet other sources consider all tidal wetlands as estuarine (Odum et al. 1984, Odum 1988). Freshwater tidal and brackish tidal wetlands are optimally developed along the lower reaches of large river systems with low gradients near the confluence with the sea. Furthermore, the floristic dissimilarities between these three classes of tidal wetlands provide a suitable justification for separate Ecological Sites.
Tidal position.
In the simplest terms, each class of tidal wetlands can be separated into two broad zones in reference to the vertical extent of the tides, 1) the “regularly” flooded zone (flooded daily on average); and 2) the “irregularly” flooded zone (flooded less than daily, i.e., only with spring tides). When projected onto the marsh profile these tidally influenced zones are commonly referred to as the “low” marsh (below Mean High Water, MHW) and the “high” marsh (above MHW). However, for the purposes of these Ecological Site Descriptions, only in the case of the salt marsh, are the high marsh and the low marsh treated separately as two different ecological sites. While, brackish and freshwater tidal sites also exhibit similar shifts in plant distribution along the intertidal profile, the geographic extent is very limited, hence the Ecological Sites of brackish tidal wetlands and freshwater tidal wetlands are considered each in their entirety, aggregating a complex of vegetation-forms over the entire tidal profile.
Tidal salt low marshes of the northeastern United States
The ecological dynamics of northeastern salt marshes are complex. Most plants have a very limited ability to grow in saline habitats except a small number of specially adapted plants, called “halophytes” (Flowers et al. 1986, Aslam et al. 2011). Furthermore, these same plants are also subjected to increasing physical harshness (decreasing redox potential and increasing salinity) associated with tidal flooding (Nichols 1920, Miller and Egler 1950, Niering and Warren 1980, Bertness and Ellison 1987). Factors responsible for the plant zonation patterns include the limiting influence of physical factors, such as tidal flooding and salinity, and additional effects of both negative and positive biotic interactions among species, like competition and facilitation, respectively (Bertness 1991a, 1991b, Bertness and Shumway 1993, Bertness and Hacker 1994, Bertness and Leonard 1997, Zhang and Shao 2013). In addition, superimposed on this complex gradient are several disturbances to the low marsh including: relative sea-level-rise, wrack, major storm events, ice-scouring/rafting, and herbivory. Furthermore, additional agents of salt marsh vegetation change are attributed directly or indirectly to human actions, including: accelerated sea-level rise, temperature warming, filling, ditching and draining, open-water-marsh-management (OMWM) and integrated-marsh-management (IMM), alteration of tidal exchange by roadcrossings, impoundment dikes, culverts, tide gates and other structures, eutrophication, sudden vegetation “dieback”, and consumer control.
Biotic interactions: stress-tolerance vs. competition trade-off and disturbance-driven facilitation
Besides coping with the changing physical limitations of the intertidal gradient in terms of stress and disturbance, plants also experience biotic interactions: competition or facilitation (Bertness and Shumway 1993, Bertness and Callaway 1994, Zhang and Shao 2013).
Competition.
The marked zonation between low and high marsh vegetation is a boundary dispute (Bertness 1992). Although Spartina alterniflora (smooth cordgrass) is capable of growing throughout the marsh, it is competitively excluded from the high marsh by turf-building perennials like Spartina patens (saltmeadow cordgrass). Spartina alterniflora dominates in the low marsh because it is one of the few halophytes that can tolerate daily flooding and halinity extremes, a habitat too stressful for Spartina patens and other high marsh perennials. Hence, it is the ecological trade-off between competition and stress tolerance as opposing species’ strategies (Grime 2006) that separates the high marsh from the low marsh.
Facilitation.
In the Northeastern US, Spartina alterniflora is the primary plant responsible for saltmarsh establishment and expansion both seaward and landward. As such, it is the foundation species that first colonizes the marsh and facilitates further marsh development (Angelini et al. 2011). Facilitation is a positive interaction that occurs when a species ameliorates stress, making the environment more favorable for other, successive species (Connell and Slatyer 1977, Bruno et al. 2003). Environmental changes that alleviate flood stress, such as higher marsh surface elevation and greater subsurface aeration that resulted from sediment trapping and the accumulation of living peat by colonizing Spartina alterniflora, lead to the succession of less-stress-tolerant but competitive dominants, Spartina patens or Juncus gerardii (Bertness and Leonard 1997, Castillo et al. 2010). Futhermore, marsh animals also contribute to marsh-building. Burrows of Uca pugnax, the Altlantic marsh fiddler, aerate the low marsh substrate, and byssal threads secreted by Geukensia demissa, the Atlantic ribbed mussel, bind and anchor Spartina alterniflora to the substate (Bertness 1992).
In summary, facilitation is common in succession under harsh physical conditions, but competition dominates under more benign physical conditions (Bertness and Shumway 1993).
Relative Sea-level Rise
Under natural conditions, salt marsh elevations are generally near or approaching a dynamic equilibrium relative to rising sea level (Friedrichs and Perry 2001, Morris et al. 2002). Historically, marsh building has occurred both landward and seaward in southern New England during times of slow [historic] rates of sea-level rise at approximately 1.0 mm•yr-1 (Nixon 1982, Donnelly et al. 2004) and marshes may appear to continue building during moderate rates of sea-level rise at ~2.5 mm•yr-1 (Bricker-Urso et al. 1989, Donnelly et al. 2004, NOAA/NOS/CO-OPS 2014). However, there is a concern that the accelerated rates of sea-level rise may eventually impose an upper limit or tipping point beyond the ability of coastal marshes to build and keep pace with sea-level rise (Bricker-Urso et al. 1989, Kirwan et al. 2010). (More details follow in the section on accelerated sea-level rise).
Wrack
The most common natural disturbance in salt marshes of the Northeastern US is the stranding of wrack. Deposited by extreme high and storm tides, the smothering action of wrack kills vegetation and renders the surface bare. In New England tidal marshes, wrack is made up mostly from decaying litter of Spartina alterniflora, and to lesser extent of Zostera marina (eelgrass). The extent and distribution of wrack varies considerably over the entire tidal marsh, yet accumulation is greater on the high marsh, especially nearest the upland border (Miller and Egler 1950, Bertness and Yeh 1994). Wrack disturbance covers only 1 to 2.4 % of the low marsh surface annually (Hartman 1984), albeit, the areas actually damaged by wrack are usually much less considering the wrack is rafted away by the regularly flooded daily tides (Valiela and Rietsma 1995).
Ice scouring and ice rafting
With increasing latitude, more northern salt marshes are progressively exposed to the effects of two different types of winter ice disturbances – ice-scouring and ice-rafting – both of which can trigger vegetation change. Ice scouring severely impacts low marsh habitats that are exposed to daily tidal water movement (Ewanchuk and Bertness 2003). During Ice rafting, ice incorporated into the low marsh can dislodge and raft away in large chunks (Ewanchuk and Bertness 2004).
Major storm events
Coastal storms have been associated with both marsh erosion and marsh building. Changes in marsh topography and position have marked influence on the distribution of marsh plants. Erosion from wave action occurs along seaward bayfronts where wave force directly attacks the low marsh and also along the creeks crossing the high marsh where strong tidal currents undermine the banks (Friedrichs and Perry 2001). Furthermore, storm related erosion is also associated with events of ice-scouring and ice rafting in northern New England (Ewanchuk and Bertness 2003). Conversely, major amounts of sediment deposition in tidal marshes are attributed to coastal storm events (Harrison and Bloom 1977, Gedan et al. 2009). Overwash events during extreme high tides and storm surges occur but are more important as marsh-building processes on the high marsh. The sedimentary record in a Rhode Island marsh revealed at least 7 hurricane-magnitude storms within the past 700 years (Donnelly et al. 2001). With climate change scenarios, the intensity of major storm activity is expected to increase in the North Atlantic (IPCC 2013a).
Herbivory
Herbivory is usually sometimes considered an insignificant factor in the structuring of saline high marsh plant communities in New England. However, pronounced Insect herbivory has been reported in patches of Salicornia sp. (Ellison 1987) and attributed to seed set reduction is about 50% for common marsh perennials. (Bertness and Ellison 1987). Yet, herbivory is more important in the low marsh, dominated by Spartina alterniflora, where triggered by indirect effects of human disturbance, significant grazing impacts have been noted by geese, snails, and crabs and insects (see also section Consumer Control).
Accelerated Sea Level Rise – vertical accretion and lateral migration
Stratigraphic evidence and tide gage information has indicated that sea-level rise in New England accelerated during the last century from about 1.0 mm yr-1 (Nixon 1982, Donnelly et al. 2004) to a rate of approximately 2.6 mm yr-1 (1938 – 2006, New London tide gage no. 8461490). The rate may be increased further to 4.5 mm yr-1 if adjusted for the latest gage data trend segment from 1987 to 2013 (Boon 2012, Sallenger et al. 2012). AR5, the Fifth Assessment Report, (IPCC 2013b) predicts that the rate of global mean sea level (GMSL) rise may conservatively increase to 4.5 mm yr-1 by mid-century then accelerate to 11 mm yr-1 or even climb as high as 16 mm yr-1 by the end of the 21st century in response to a decadal warming trend ranging from 0.08 to 0.14 °C. These recent trends and projections in accelerated sea level rise due to climate change (global warming ) (Varekamp and Thomas 1998, Boon 2012, IPCC 2013b) has caused concern about (1) whether vertical salt marsh accretion can keep pace (Reed 1995, Kirwan et al. 2010), (2) whether the horizontal development of marshes has sufficient neighboring space upon which to laterally migrate. Any significant changes in the vertical or lateral extent of the tidal marsh can lead to a state change in the vegetation. It is likely that the Northeast will experience an increase in the extent of low marsh and intertidal flats due to accelerated sea-level rise (Tiner 2013).
Vertical accretion.
Vertical stability of the tidal marsh’s surface elevation is achieved when wetland surface elevation gains are approximately the same rate of sea level rise. Elevation gains are driven by the accumulation of organic matter (i.e., “peat”, the persistent buildup of belowground vegetation growth and litter) and the accumulation of inorganic sediment, minus any losses attributed to erosion, subsidence (i.e., decomposition, and compaction) (Morris et al. 2002, Cahoon et al. 2006, Fagherazzi et al. 2012). To precisely document vertical processes, and evaluate marsh resilience or vulnerability and vegetation state changes, to rising sea level at the site-specific level, a network of “Surface-Elevation Table and Marker Horizon” (SET-MH) set-ups exist throughout New England and elsewhere (Cahoon et al. 1999, 2006). Depending on the location and geomorphic setting, the contribution of vertical processes of marsh accretion varies. In New England, peat formation dominates vertical accretion (Bricker-Urso et al. 1989, Turner 2011). Low marshes in the northeast, are accreting 1.5 times faster than the high marsh (Bricker-Urso et al. 1989, Hartig et al. 2002). Predictions of warmer temperatures and longer growing season may also enhance plant productivity 25 percent more in the low marsh (Charles and Dukes 2009, Kirwan and Megonigal 2013). In turn, greater aboveground biomass also accelerates sedimentation (Friedrichs and Perry 2001, Morris et al. 2002). Therefore, greater productivity and sedimentation rates suggest that Spartina alterniflora will act as a stabilizing mechanism in the face of sea-level rise (Fagherazzi et al. 2012, Kirwan and Megonigal 2013). Yet, as sea level continue to rise, organic matter accrual is eventually limited by “drowning’ when increasing tidal flood levels no longer allow plant growth or survival (Morris et al. 2002, Kirwan et al. 2010) reducing any “elevation capital” to buffer further increases in the rate of sea level rise (Cahoon and Guntenspergen 2010). In fact, due to low sediment concentrations in the Northeast, these peat-based marshes may be less resilient to sea level increases (Gedan and Bertness 2010, Weston 2014). Models predict a threshold sea level rise rate of 5 mm yr-1 for the peat marshes in the Plum Island Estuary, MA assuming suspended sediment concentrations remain low (Kirwan et al. 2010). In the Northeast, evidence of submerging marshes is revealed by shifts and disappearance of vegetation currently observed in SW Long Island Sound (Tiner et al. 2006), Jamaica Bay (Hartig et al. 2002), and Cape Cod (Smith 2009).
Lateral migration.
Another aspect of sea level rise is lateral migration, where salt marshes expand into adjacent low lying wetlands, up rivers, and onto uplands. This process, known as “marine transgression”, has continued at varying rates since the last glaciation (Redfield 1972, Niering and Warren 1980, van de Plassche 1991). Spatially explicit models showing future marsh migration locales are being used for insight into the into coastal planning and resource management (Fagherazzi et al. 2012). Sea Level Affecting Marshes Model (SLAMM) is a spatial GIS model that simulates the dominant processes involved in wetland conversions and shoreline modifications during long-term sea level rise (Mcleod et al. 2010). Map distributions of wetlands are predicted under conditions of accelerated sea level rise, and results are summarized in tabular and graphical form; http://warrenpinnacle.com/prof/SLAMM/index.html.
The Nature Conservancy’s Coastal Resilience Project includes a GIS tool that identifies coastal areas suitable for migration, as well as potential barriers to migration, and the relative importance of different marshes based on their potential to provide ecosystem services. In New England, the migration potential has been mapped for marshes in Long Island and Connecticut http://coastalresilience.org/geographies/new-york-and-connecticut ; http://maps.coastalresilience.org/nyct/#, (Hoover et al. 2010).
Temperature warming
In addition to driving accelerated sea level rise (IPCC 2013b), temperature warming also subtly effects the composition and distribution of salt marsh vegetation. In response to higher summer temperatures and extended growing season, Spartina alterniflora (smooth cordgrass) is expected to increase productivity 25% (Charles and Dukes 2009) and competitiveness (Emery et al. 2001).
Filling
Filling coastal marshes for land reclamation projects ranks among the most radical and greatest impact because it almost always results in permanent habitat conversion and loss. Losses due to dredge and fill operations were common to improve navigation and expand seaports and marinas (Rozsa 1995, Tiner 2013). Some coastal tidal marsh loss assessments have been estimated at 30% for the Long Island Sound from 1880 to 1970 (Dreyer et al. 1995) and 37% for parts of Maine, New Hampshire, Massachusetts, and Rhode Island (from roughly 150 to 200 years ago) (Bromberg and Bertness 2005). Coastal marsh losses in the greater Boston is estimated at 81% (Bromberg and Bertness 2005). Although these estimated losses are proportionately greater for the salt high marsh, some salt low marsh is included.
Ditching
Historically, northeastern salt marshes have been extensively ditched and drained (Miller and Egler 1950, Redfield 1972, Niering and Warren 1980, Nixon 1982). The most intensive ditching occurred in the 1930s, by the Civil Works Administration and Works Progress Administration, for the purposes of mosquito control and employment (Rozsa 1995, Gedan et al. 2009). The type of ditch network most commonly excavated in the northeast was a pattern of successive straight parallel ditches running from upland to open water (sometimes called grid ditches if cross-ditching was evident). Ditching is a major hydrological alteration to the salt high marsh (but less so to the low marsh). Ditches circumvent the natural drainage system with a network of shortcuts that increase the marsh surface area enhanced by tidal flooding and drainage that would not ordinarily be affected except for astronomical highs (Tonjes 2013). Ditches create low marsh habitats supporting Spartina alterniflora along ditch banks and in aggraded ditches (Miller and Egler 1950). Ditches appear to have expanded and accelerated sudden vegetation die-backs by enlarging the susceptible low marsh habitat (Alber, Swenson, Adamowicz, & Mendelssohn, 2008; Coverdale, Bertness, & Alteiri, 2013). Enhanced ditch circulation translates to potential nutrient enrichment and eutrophication (Koch and Gobler 2009, Tonjes 2013) favoring either Spartina alterniflora or the invasive Phragmites australis in less waterlogged, less haline situations (Amsberry et al. 2000, Tiner 2013).
Eutrophication (nutrient loading)
Eutrophication, particularly nitrogen inputs originating from shoreline development and removal of adjacent vegetation buffers is contributing to shifting changes in the salt marsh vegetation (Silliman et al. 2009, Gedan et al. 2011). Since most salt marsh plants are nitrogen limited, eutrophication disrupts the competitive hierarchy/stress and disturbance gradients associated with the typical zonation of the New England salt marsh (Emery et al. 2001). This process enables the expansion of Spartina alterniflora into the high marsh displacing Spartina patens and the proliferation of Phragmites australis at the upper border displacing Juncus gerardii (Levine et al. 1998, Bertness et al. 2002). Although nitrogen additions can increase aboveground biomass, eutrophication reduces the nutrient seeking belowground root and rhizomatous biomass volume and strength, ultimately leading to less peat accretion, plant collapse, and potentially drowning (Valiela et al. 1976, Turner et al. 2009, Gedan et al. 2011, Deegan et al. 2012). However some nitrogen enriched salt marshes already effected may not exhibit reduced biomass and elevation loss (Anisfeld and Hill 2012). Eutrophication can trigger consumer control by herbivorous insects which can suppress primary production by as much as 50 percent in the low marsh (Bertness and Silliman 2008).
Open Marsh Water Management/Integrated Marsh Management
Open water marsh management (OMWM) was a response of management to address the long-term failures of ditching to more effectively control mosquitos. The concept of OMWM is to re-create irregularly flooded saltmarsh pools on the high marsh that were eliminated by ditching to provide habitat for mosquito-eating fish, notably the mummichog, Fundulus heteroclitus (Rochlin et al. 2012a). Integrated marsh management (IMM) is a multi-purposeful approach to salt marsh ecosystem management that uses best management practices of salt marsh restoration while also incorporating various mosquito control methods (Rochlin et al. 2012b). The main idea of IMM is to restore or emulate native salt marsh conditions using mostly geomorphic alterations to achieve tidal flow restoration, vegetation management, and aspects of OMWM. IMM /OMWM projects are typically associated with the irregularly flooded high marsh and only incidentally involve low marsh. However, since IMM /OMWM projects tend to be highly physically disruptive, a systematic program is strongly recommended to first evaluate and always monitor the impacts and benefits to the vegetation (Potente 2007).
Tidal restriction and exclusion due to causeways, impoundment dikes, culverts, and tide gates
Tidal restriction limits coastal marshes from essential marine tidal influences, which alters the physical and biotic conditions on the marsh leading to a state change in the vegetation. Tidal restrictions, for example, dikes and impoundments, are constructed across coastal wetlands and are more an issue with the high marsh (for more details, see tidal salt high marsh ecological site), but incidentally include low marsh. Marsh habitats behind constructed dikes are effectively isolated from marine influence and degrade due to reduced accretion, lowered water tables, and reduced salinities. Reduced salinities result in the conversion to brackish marsh species (Roman et al. 1984) such as cattails, (Typha latifolia and Typha x glauca) and bulrushes (Schoenoplectus spp.) as well as the common reed (Phragmites australis or possibly the native genotype, Phragmites australis ssp. americanus). In the most extreme conditions, if tidal flow is completely severed (closed) relative to freshwater inputs, a freshwater marsh system or open water impoundment results (Roman et al. 1984, Warren et al. 2002, Tiner 2013).
Marsh “dieback”
Marsh dieback, also known as “sudden vegetation dieback”, SVD, is a condition that has been described as the loss or death of emergent vegetation in salt marsh ecosystems. It was originally characterized by standing dead vegetation only, characterized exclusively as not over-grazed, wracked, ice-sheared, etc. (Alber et al. 2008). Marsh dieback is most commonly associated with the death of Spartina alterniflora (smooth cordgrass) of the low marsh, although exceptions exist on the tidal wetlands of Cape Cod, MA (Smith 2009). Marsh dieback is particularly troubling marsh because it impacts the developing edge of Spartina alterniflora which is critical to marsh development (Gedan et al. 2011) While the nature of marsh dieback is clear, what causes dieback is poorly understood and controversial (Linthurst 1979, Alber et al. 2008, Craft et al. 2008, Holdredge et al. 2009, Smith 2009, Elmer and Marra 2011, Anisfeld and Hill 2012). Dieback has been associated with drought in the Southeast US and the Gulf, but not in the Northeast US (Alber et al. 2008). Conditions commonly associated with dieback areas are changes in soil conditions due to more frequent or prolonged flooding such as lower redox and anaerobic or anoxic (oxygen-deficient) conditions (Alber et al. 2008). For example, the dieback process has been associated with accelerated sea-level rise (Hartig et al. 2002, Smith and Carullo 2007, Smith 2009, Tiner 2013) and waterlogged areas as a result of ditch-plugging (Vincent et al. 2013). Fungal pathogens such as Fusarium spp.are also suspected (Elmer and Marra 2011). Top-down consumer control that results in unchecked herivory by the purple marshcrab, Sesarma reticulatum, is also implicated in dieback events especially on Cape Cod. Symptoms of SVD there are stark, completely denuded low marshes and ditch habitats lacking any Spartina alterniflora (Holdredge et al. 2009, Altieri et al. 2012, Coverdale et al. 2013b). Marsh dieback is particularly troubling because it impacts the developing edge of Spartina alterniflora which is critical to marsh development (Gedan et al. 2011). It is likely there is no single reason for dieback, rather a condition due to several stressors.
Consumer Control (subsidized or unchecked herbivory)
Among the impacts to salt marshes attributed to human activity, less understood are vegetation losses due to indirect and subtle factors, such as “consumer control”. Triggered by human disturbance, herbivory by plant consumers either goes unchecked or gets subsidized, resulting in significant vegetation loss in areas where plant consumers were not historically important (Power 1992, Pace et al. 1999, Bertness and Silliman 2008). Examples of consumer control triggered by human disturbance, include significant grazing impacts by geese, snails, and crabs and insects, mostly upon Spartina alterniflora, especially in the low marsh.
Salt marsh eutrophication from enriched discharges and runoff, enhance insect herbivory by grasshoppers, Conocephalus spartinae, and leafhoppers, Prokelisia marginata, reducing the vegetation by nearly 60% (Bertness et al. 2008, Sala et al. 2008).
Fueled by fertilizer subsidies to ag fields and golf courses, inflated populations of gregarious and migratory geese occur at nuisance levels; such that Snow Geese, Chen [Anser] caerulescens, have denuded expansive marshes in the Subartic (Jefferies et al. 2006) and to a lesser extent southeastern US (Smith and Odum 1981), and Canada Geese, Branta Canadensis, overgraze saltmarshes throughout New England (Buchsbaum et al. 1981, Teal 1986).
On Cape Cod and perhaps elsewhere, recreational fishing has depleted predatory fish, allowing explosive increases in the populations of the native, herbivorous purple marsh crab, Sesarma reticulatum, that completely denude the high marsh ditches and low marsh of Spartina alterniflora (Holdredge et al. 2009, Altieri et al. 2012, Coverdale et al. 2013b). However, it appears that the invasion of the predatory European green crab, Carcinus maenas, may keep the populations of Sesarma in check and reverse the effects over-grazing (Bertness and Coverdale 2013).
The regularly flooded condition anticipated with accelerated sea-level rise is expected to heighten the grazing pressure of the long-introduced common periwinkle, Littorina littorea, (Bequaert 1943) on Spartina alternifora along high marsh creekbanks and the low marsh (Bertness 1984, Tyrrell et al. 2008).
State Transitions and Ecological Site Transitions
Several transitions occur that transform the typical salt low marsh reference condition. Two are state transitions that result from dieback and tidal restriction. Both states are subject to restoration actions. These state transitions are featured by appropriate numbers at the core of the state-and-transition model and within appropriate sections of the corresponding narrative. Four additional transitions are warranted that result in crossovers to entirely different ecological sites. These are external transitions that lead to, or, lead from, other ecological sites. (Unfortunately, current database limitations do not easily account for levels beyond a state transition. Therefore, an alternative solution is to simply include external transitions in the diagram without numbers or boxes and add a short description in the narrative.) External transitions to other ecological sites are nonetheless important to the ecological dynamics of the salt marsh ecosystem. These external ecological site transitions include highly significant yet commonplace processes or disturbances, and are listed as follows:
Transition to irreversibly Filled ecological sites of various types
The deposition of fill, human-transported material, to the extent of excluding the tides, creates non-tidal and upland ecological sites of various types.
Transition from Tidal Salt High Marsh ecological site to Tidal Salt Low Marsh ecological site
A shift up and landward in the regularly flooded (flooded daily) intertidal zone associated with accelerated sea level rise results in greater flood stress leading to the displacement of the Tidal Salt High Marsh ecological site to the Tidal Salt Low Marsh ecological site, dominated by Spartina alterniflora (smooth cordgrass).
Transition from Tidal Brackish Wetland ecological site to Tidal Salt High Marsh ecological site.
Increasing flood stress associated with accelerated sea level rise results in the loss of brackish species such as Lilaeopsis chinensis (eastern glasswort) to monotypic stands of Spartina alterniflora (smooth cordgrass).
Transition from Tidal Salt Low Marsh ecological site to Tidal Salt Flats ecological site
Accelerated sea level rise is converting the seaward edge of the intertidal zone into an intermittently exposed Tidal Salt Flats ecological site occasionally populated by marine alage Ulva spp. (sea lettuce), Enteromorpha spp. (hollow greenweeds)and occasionally loose mats of Fusus vesiculosus (rockweed) and Ascophyllum nodosum (knotted wrack).
The ecology of tidal wetlands of the Northeastern United States is well studied. Accounts of the vegetation and dynamics reported here are derived largely from the literature and augmented by field sampling.
The information contained in this Ecological Site Description (ESD) and State and Transition Model (STM) were developed using historical data, professional experience, and scientific studies. The information presented is representative of a very complex set of plant communities. Not all scenarios or plants may be included. Key indicator plants, animals and ecological processes are described to inform land management decisions.
Additional information for Tidal Low Salt Marsh R144AR001CT is based on original field observations and relevé plots sampled. The field work was conducted by the staff of State Soil Office 12-TOL, and NRCS Ecologist. Additional relevé plots were provided courtesy of VegBank, the CT Natural Diversity Database (NDDB, Natural Heritage Program), and CT Department of Energy and Environmental Protection, (CT-DEEP) Wildlife Division.
State and transition model
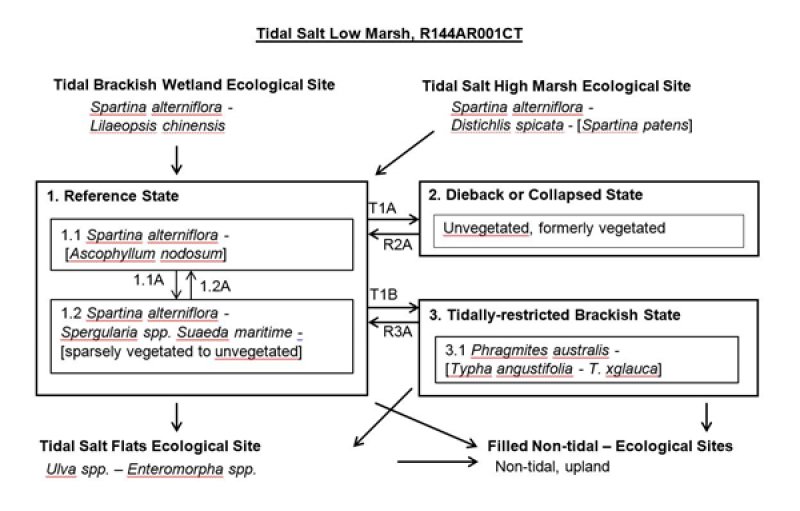
Figure 10. Tidal Slat Low Marsh - R144AR001CT
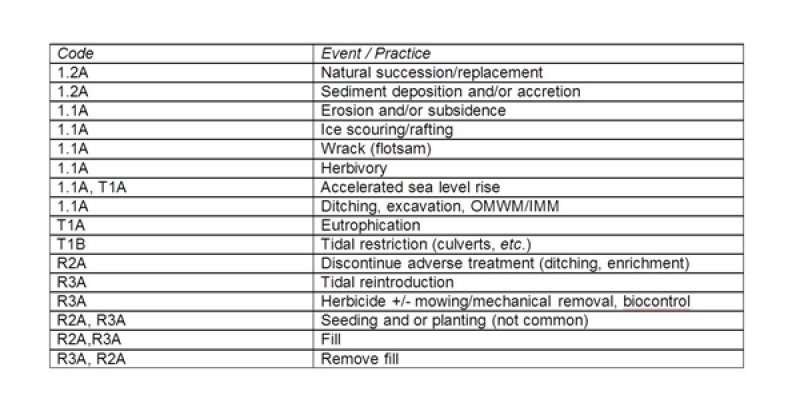
Figure 11. Tidal Salt Low Marsh - R144AR001CT
More interactive model formats are also available.
View Interactive Models
More interactive model formats are also available.
View Interactive Models
Click on state and transition labels to scroll to the respective text
Ecosystem states
State 2 submodel, plant communities
State 3 submodel, plant communities
State 1
Reference
The reference state is a typical Northeastern US tidal salt low marsh, characterized by (1.1) Spartina alterniflora (smooth cordgrass) with occasional algae, (1.2) sparsely vegetated Spartina alterniflora and other salt tolerant forbs, and (1.3) mostly algae. Biomass values are from Nixon and Oviatt (1973). Note that some transitions away from the reference site lead to different states and still other more severe transitions occur that transform the typical salt high marsh reference condition into completely different ecological sites. A brief synopsis of these external transitions and ecological sites are located near the end of the previous section on ecological dynamics.
Community 1.1
Saltmeadow cordgrass – [knotted wrack] (Spartina alterniflora – [Ascophyllum nodosum])

Figure 12. Spartina alterniflora

Figure 13. S. alterniflora - mineral soil
This community type is dominated by nearly a monospecific stand of Spartina alterniflora (smooth cordgrass) in the tall form growing over a meter tall. Occasionally, it may also contain mats of the marine algae species Ascophyllum nodosum (knotted wrack) and Fucus vesicularis (rockweed). Biomass values from Nixon and Oviatt (1973).
Figure 14. Annual production by plant type (representative values) or group (midpoint values)
Table 5. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | – | – | – | – |
| >0.5 <= 1 | – | – | 0-100% | – |
| >1 <= 2 | – | – | – | – |
| >2 <= 4.5 | – | – | – | – |
| >4.5 <= 13 | – | – | – | – |
| >13 <= 40 | – | – | – | – |
| >40 <= 80 | – | – | – | – |
| >80 <= 120 | – | – | – | – |
| >120 | – | – | – | – |
Community 1.2
Smooth cordgrass – sandspurry – seablite (S. alterniflora - Spergularia spp. – Suaeda maritima

Figure 15. Spergularia marina

Figure 16. Salicornia depressa
This community is generally open and sparsely vegetated but dominated by Spartina alterniflora (smooth cordgrass) and other flood-tolerant forbs Spergularia salina (salt sandspurry)and Spergularia canadensis (Canadian sandspurry), and Suaeda maritima (herbaceous seablite or seepweed) or ocassionally Salicornia spp. (glasswort). Occasionally, mats of marine algae Ascophyllum nodosum (knotted wrack) and Fucus vesicularis (rockweed) are found.
Figure 17. Annual production by plant type (representative values) or group (midpoint values)
Table 6. Canopy structure (% cover)
| Height Above Ground (ft) | Tree | Shrub/Vine | Grass/ Grasslike |
Forb |
|---|---|---|---|---|
| <0.5 | – | – | – | – |
| >0.5 <= 1 | – | – | 0-100% | 0-50% |
| >1 <= 2 | – | – | – | – |
| >2 <= 4.5 | – | – | – | – |
| >4.5 <= 13 | – | – | – | – |
| >13 <= 40 | – | – | – | – |
| >40 <= 80 | – | – | – | – |
| >80 <= 120 | – | – | – | – |
| >120 | – | – | – | – |
Pathway 1.1A
Community 1.1 to 1.2
![Saltmeadow cordgrass – [knotted wrack] (Spartina alterniflora – [Ascophyllum nodosum])](https://edit.jornada.nmsu.edu/uploads/med/es/pc/7316.jpg)

Agents of disturbance such as wave erosion, ice-scouring can open the site to other flood tolerant forbs.
Pathway 1.2A
Community 1.2 to 1.1

![Saltmeadow cordgrass – [knotted wrack] (Spartina alterniflora – [Ascophyllum nodosum])](https://edit.jornada.nmsu.edu/uploads/med/es/pc/7316.jpg)
Typical vegetation development can lead to greater density of Spartina alterniflora and fewer forbs.
State 2
Dieback or collapsed
Whatever the cause, this state is characterized by the former occupation and subsequent dieback or sudden vegetation dieback (SVD) of Spartina alterniflora smooth cordgrass.
Community 2.1
unvegetated (formerly occupied by smooth cordgrass (Spartina alterniflora))
Formerly populated by vital stands of Spartina alterniflora (smooth cordgrass), dieback sites are unvegetated.
State 3
Tidally-restricted State
Marsh surface modifications and engineered structures, e.g., filled causeways, constricted bridges, undersized and improperly emplaced culverts, cause tidal restrictions (Roman 2012, Tiner 2013). Tidal restrictions reduce tidal flooding, lower salinity, and may lower the water table, changing the character of the salt marsh which prompts a state change in the vegetation. The type of replacement vegetation depends upon the nature of the restriction and the local drainage pattern. Open restrictions, such as culverts, are common and have less impact then closed restrictions that prohibit tidal exchange entirely (Crain et al. 2009).
Community 3.1
Common reed - cattails (Phragmites australis - Typha spp.) tidally-restricted marsh

Figure 18. Phragmites australis
Due to tidal restrictions, reduced salinities in the range of 0.5 to 18 ppt (0.8 – 28.1 dSm-1) result in in the conversion of salt low marsh marsh species to more competitive brackish marsh species, such as Typha angustifolia (narrow-leaved cattail) and Typha x glauca (hybrid cattail), and Schoenoplectus spp. (bulrushes) as well as invasive Phragmites australis (common reed), or even possibly the native genotype, Phragmites australis ssp. americanus (American reed)(Crain et al. 2004, 2008). The vegetation is typically a dense mosaic often dominated by a single species with scattered occurrences of other common brackish species, like Hibiscus moscheutos (crimson-eyed rose mallow), Pluchea odorata var. succulenta (sweetscent), Mikania scandens (climbing hempvine), and Solidago sempervirens (seaside goldenrod). Eutrophication may favor the establishment of invasive Phragmites (Amsberry et al. 2000).
Transition T1A
State 1 to 2
Complete death of low marsh vegetation leaving an unvegetated state.
Transition T1B
State 1 to 3
Dikes or causeways with engineered structures with open restrictions, e.g., culverts, undersized tide gates, etc., that restrict but do not exclude the tidal flooding of the marsh, may ultimately change the vegetation to a brackish state, often supporting the invasive Phragmites (common reed).
Restoration pathway R2A
State 2 to 1
Restoration occurs on mineral soil that is exposed or transported (Altieri et al. 2013). Spatina alterniflora (smooth cordgrass) may be planted, although this is more common for living shorelines techniques (Erdle et al. 2008). More common are ecologically-engineered “self-design” approaches (Weinstein et al. 2001) where Spartina alterniflora seeds itself.
Conservation practices
| Wetland Wildlife Habitat Management | |
|---|---|
| Wetland Restoration |
Restoration pathway R3A
State 3 to 1
Restoration involves removing or fixing the tidal restrictions (e.g., culvert size, location, invert, etc.) to tidal flood elevations corresponding to salt tidal low marsh ecological site conditions.
Conservation practices
| Wetland Wildlife Habitat Management | |
|---|---|
| Wetland Restoration |
Additional community tables
Table 7. Community 1.1 plant community composition
| Group | Common name | Symbol | Scientific name | Annual production (lb/acre) | Foliar cover (%) | |
|---|---|---|---|---|---|---|
|
Grass/Grasslike
|
||||||
| 1 | Tidal salt low marsh | 400–12000 | ||||
| smooth cordgrass | SPAL | Spartina alterniflora | 3500–12000 | 20–100 | ||
Table 8. Community 1.2 plant community composition
| Group | Common name | Symbol | Scientific name | Annual production (lb/acre) | Foliar cover (%) | |
|---|---|---|---|---|---|---|
|
Grass/Grasslike
|
||||||
| 1 | S.alterniflora / Forb | 0 | ||||
| smooth cordgrass | SPAL | Spartina alterniflora | 3500–12000 | – | ||
|
Forb
|
||||||
| 2 | S.alterniflora / Forb | 0 | ||||
Interpretations
Animal community
Wildlife Habitat General Information
Wildlife use the salt tidal marsh in a variety of ways as either resident or migratory species. The most conspicuous are the macrofauna, which include the many invertebrates, including insects and spiders, and molluscs, crabs and snails, as well as fish, reptiles, birds, and mammals.
Invertebrate Habitat
Since the tidal environment is alternately flooded and exposed, tidal marshes provide habitat conditions that range from somewhat terrestrial to suitably aquatic (Tiner, 2013). Terrestrial invertebrates are dominated by insects and spiders that thrive in the high marsh and access the low marsh at low tides. Whereas, marine invertebrates that dominate the low marsh, intertidal and subtidal flats are characterized by and assortment of snails, crabs, molluscs and worms. Swimming invertebrates such as shrimp (Palaemonetes) move in and out with the tides.
There are many kinds of insects and spiders found in the salt marsh, mostly confined to higher elevations (Teal, 1986). The most conspicuous insects are, of course, are the biting insects including the salt marsh mosquitos (Ochlerotatus [formerly Aedes] sollicitans, O. cantator), greenheads (Tabanus nigrovittatus), and no-see-ums (Culicoides spp.). Insect herbivores include chewers and suckers. The predominant chewing insect are grasshoppers (Conocephalus spp.), katydids (Orchelimum spp.) and thrips (Anaphothrips spp.). Sucking insects are much more abundant. The predominant sucking insect is the planthopper (Prokelisia marginata). Others include plant bugs (Miridae, Trigontylus), aphids (Aphidae), scale insects (Coccoidea) and fly larvae of many species (e.g., Chloropids, Dolichopodids, and Ephydrids). Insect predators include many spiders like web-weavers (Grammonota inornata and Dictyna roscida), sac spider (Clubiona maritima), and the wolf spider (Pardosa distincta). Additional information may be found in Tiner (2013), Teal (1986), and Nixon (1982).
Typically, snail species more are common to the high marsh whereas most crab species inhabit the low marsh. Common snails include the common periwinkle (Littorina littorea) and the rough periwinkle (Littorina saxatilis) and the pulmonate salt marsh snail (Melampus bidentatus). These snail feed by scraping off algae and detritus from tidal marsh surfaces (Teal, 1986). The salt marsh snail is often seen climbing the stalks of Spartina alterniflora during the flood tide to avoid inundation and predation from fishes and crabs (Tiner, 2013).
Burrowing crabs leave conspicuous holes at the edges of creeks and ditches. The common fiddler crab (Uca pugnax) prefers burrowing and deposit-feeding in the stable peaty muck of the low marsh which potentially aerates the marsh soils at ebb tide and boosts the growth and productivity of Spartina alterniflora (Bertness, 1985). The native herbivorious purple marsh crab (Sesarma reticulatum) has been implicated in low marsh dieoff, due to overgrazing pressures in some marshes where the populations were not held in check by predatory fish that were removed by overfishing(Holdredge et al., 2009; Altieri et al., 2012; Coverdale, Bertness, et al., 2013). Ironically, the predatory nonnative European green grab, Carcinus maenas, may keep the populations of the purple marsh crab in check and reverse the effects over-grazing (Bertness and Coverdale, 2013).
Fish Habitat
Tidal marshes provide sheltered shallow water habitat, nursery and foraging habitat for numerous fishes (Teal, 1986; Tiner, 2013). Several small fishes that spend most of their lives within high marsh pools and in creeks during the advancing tides are : mummichog (Fundulus heteroclitus), Sheepshead minnow (Cyprinodon variegatus), Atlantic silverside (Menidia menidia), Striped killifish (Fundulus majalis), Four-spined stickleback (Apeltes quadracus), and Three-spined stickleback (Gasterosteus aculeatus). Common fishes that use the tidal marsh mainly as a nursery area are: Winter flounder (Pseudopleuronectes americanus), Tautog (Tautoga onitis), Sea bass (Centropristes striata), Alewife (Alosa pseudoharengus), Menhaden (Brevoortia tyrannus), Bluefish (Pomatomus saltatrix), and Mullet (Mugil cephalus). Additional species may be found in Tiner (2013).
Reptile Habitat
Excepting sea-turtles, which are essentially marine, the diamondback terrapin (Malaclemys terrapin) commonly feeds in the high marsh ditches and creeks and low marsh. Terrapins have declined due to coastal development and collecting pressures (Teal, 1986).
Bird Habitat
One of the most widely recognized values of salt marshes is their support of habitat for birds. Tidal wetlands provide a year-round home for resident birds and serve as important stopover areas for breeding, feeding and overwintering of migratory birds along the Atlantic flyway. (Teal, 1986; Tiner, 2013). Those birds in-and-around tidal marshes include (but not limited to), the marsh specialists that live and feed in the salt marshes: Willet (Catoptrophorus semipalmatus), Clapper Rail (Rallus longirostris), Saltmarsh sharp-tailed sparrow (Ammodramus caudacutus), Seaside sparrow (Ammodramus maritimus); waders that stalk small fishes and crustaceans: Great blue heron (Ardea herodias), Glossy ibis (Plegadis falcinellus), Great egret (Ardea alba), Snowy egret (Egretta thula), shorebirds probe for invertebrates: Killdeer (Charadrius vociferus), Greater yellowlegs (Tringa melanoleuca), Lesser yellowlegs (Tringa flavipes), Spotted sandpiper (Actitis macularia), and other sandpipers of the Calidrid tribe (Calidris spp.); seabirds that may feed in open water on the marsh: gulls (Laridae), terns (Sternidae); waterbirds: Double-crested cormorant (Phalacrocorax auritus); waterfowl: Mute swan (Cygnus olor), Canada goose (Branta canadensis) that graze on Spartina alterniflora, numerous ducks of the Anitini tribe that sieve seeds (e.g., Black duck (Anus rubripes) and Mallard (Anus platyrhynchos)); marsh generalists: Song sparrow (Melospiza melodia), Red-winged blackbird (Agelaius phoeniceus); and other birds such as: Northern harrier (Circus cyaneus), Short-eared owl (Asio flammeus), Common grackle (Quiscalus quiscula). The relatively high diversity of birds of all other types on the high marsh is considered an "edge effect" of the marsh-upland ecotone where terrestrial birds do mix with birds more typical of the estuary (Nixon, 1982).
Mammal Habitat:
A few small mammals are associated directly with the salt marsh, namely, Muskrat (Ondatra zibethicus), meadow vole (Microtus pennsylvanicus), Racoon (Procyon lotor) and shrews (Sorax spp.). Muskrats and voles eat the marsh vegetation and their presence is evidenced by their runways. Muskrats prefer brackish marshes and are common in reed marshes composed of cattails (Typha spp.) and/or common reed (Phragmites australis). Shrews and raccoons prey mainly on small animals, especially invertebrates.
The conservation significance of tidal salt marshes as wildlife habitat are evidenced by the high priority given in the various northeastern state’s “Comprehensive Wildlife Conservation Strategy”. These wildlife action plans proactively assess the health of each state’s wildlife and habitats, identify the problems they face, and outline the actions that are needed to conserve them over the longterm. All 50 States and five U.S. territories developed a State Wildlife Action Plan (SWAP) in 2005 and are expected to update in 2015. To see more and select a state go to http://www.teaming.com/state-wildlife-action-plans-swaps .
Hydrological functions
The hydrology of the salt marsh ecosystem is dominated by tidal exchange with the Atlantic Ocean including Long Island Sound and Narragansett Bay. The trend in the highest astronomical range are in two directions - northward toward ME (Portsmouth, NH, 2.864 m great tidal range [GT]) and east toward New York City (Kings Point, NY 2.378 m great tidal range [GT]), with lessor tidal amplitudes at intervening stations (Wood Hole, MA, ).67 m great tidal range [GT]).
Recreational uses
Many recreational activities, such as boating, hiking, wildlife observation, painting, and tourism including sporting activities like hunting, fishing, and crabbing, provide recreational opportunities for the public as well as economic opportunities for private landowners. Town landings, boat launches, and both public and private parks and beaches are located nearby. Recreational boating, tours and fishing is available throughout the coastal region. Bird watching along the coast has become increasingly popular with the public.
Wood products
n/a
Other products
n/a
Supporting information
Inventory data references
The data contained in this document is derived from the analysis of field inventories (relevé plots and reconnaissance notes collected by MLRA Soil Survey Office 12-TOL. Five high intensity plots were conducted for the reference state during the NRCS halinty Phase II / ESD Project. This information was supplemented by plot data from the Connecticut Department of Energy and Environmental Protection (CT-DEEP) Wildlife Unit, New York Natural Heritage Program retreived through VegBank (http://vegbank.org/vegbank/index.jsp) and National Park Service plots collected at Hatches Harbor by the principal author.
Type locality
| Location 1: Fairfield County, CT | |
|---|---|
| Latitude | 41° 9′ 50″ |
| Longitude | -73° 9′ 33″ |
| General legal description | Great Meadows Marsh Stewart B. McKinney Refuge. Backmarsh along creek next to parking lot and signage along Long Beach Blvd., Bridgeport, CT. |
| Location 2: Middlesex County, CT | |
| Latitude | 41° 17′ 14″ |
| Longitude | -72° 19′ 26″ |
| General legal description | Low Marsh adjacent to and south of State boat launch of Smith Neck Rd., Old Lyme, CT (behind Great Island). |
| Location 3: Newport County, RI | |
| Latitude | 41° 29′ 21″ |
| Longitude | -71° 23′ 46″ |
| General legal description | Fox Hill salt marshes aka Fort Getty salt marsh. Nice example of salt marsh toposequence from low marsh, high marsh meadow, and upper marsh border. |
Other references
Alber, M., Swenson, E. M., Adamowicz, S. C., & Mendelssohn, I. A. 2008. Salt marsh dieback: an overview of recent events in the US. Estuarine, Coastal and Shelf Science, 80(1): 1–11.
Altieri, A. H., Bertness, M. D., Coverdale, T. C., Axelman, E. E., Herrmann, N. C., & Szathmary, P. L. 2013. Feedbacks underlie the resilience of salt marshes and rapid reversal of consumer driven die-off. Ecology. 94(7): 1647-1657.
Altieri, A. H., Bertness, M. D., Coverdale, T. C., Herrmann, N. C., & Angelini, C. 2012. A trophic cascade triggers collapse of a salt-marsh ecosystem with intensive recreational fishing. Ecology, 93(6): 1402–1410.
Amsberry, L., Baker, M. A., Ewanchuk, P. J., & Bertness, M. D. 2000. Clonal integration and the expansion of Phragmites australis. Ecological Applications, 10(4): 1110–1118.
Anisfeld, S. C., & Hill, T. D. 2012. Fertilization Effects on Elevation Change and Belowground Carbon Balance in a Long Island Sound Tidal Marsh. Estuaries and Coasts, 35(1): 201–211.
Aslam, R., Bostan, N., Nabgha-e-Amen, M. M., & Safdar, W. 2011. A critical review on halophytes: Salt tolerant plants.
Bequaert, J. 1943. The genus Littorina in the western Atlantic. Johnsonia, 1(7): 1–27.
Bertness, M. D. 1984. Habitat and Community Modification by An Introduced Herbivorous Snail. Ecology, 65(2): 370–381.
Bertness, M. D. 1991a. Interspecific Interactions among High Marsh Perennials in a New England Salt Marsh. Ecology, 72(1): 125–137.
Bertness, M. D. 1991b. Zonation of Spartina Patens and Spartina Alterniflora in New England Salt Marsh. Ecology, 72(1): 138–148.
Bertness, M. D. 1992. The Ecology of a New England Salt Marsh. American Scientist, 80(3): 260–268.
Bertness, M. D., & Callaway, R. 1994. Positive interactions in communities. Trends in Ecology & Evolution, 9(5): 191–193.
Bertness, M. D., & Coverdale, T. C. 2013. An invasive species facilitates the recovery of salt marsh ecosystems on Cape Cod. Ecology.
Bertness, M. D., Crain, C. M., Holdredge, C. T., & Sala, N. C. 2008. Eutrophication and Consumer Control of New England Salt Marsh Primary Productivity. Conservation Biology, 22(1): 131–139.
Bertness, M. D., & Ellison, A. M. 1987. Determinants of Pattern in a New England Salt Marsh Plant Community. Ecological Monographs, 57(2): 129–147.
Bertness, M. D., Ewanchuk, P. J., & Silliman, B. R. 2002. Anthropogenic modification of New England salt marsh landscapes. Proceedings of the National Academy of Sciences, 99(3): 1395–1398.
Bertness, M. D., & Hacker, S. D. 1994. Physical Stress and Positive Associations Among Marsh Plants. The American Naturalist, 144(3): 363–372.
Bertness, M. D., & Leonard, G. H. 1997. The role of positive interactions in communities: lessons from intertidal habitats. Ecology, 78(7): 1976–1989.
Bertness, M. D., & Shumway, S. W. 1993. Competition and Facilitation in Marsh Plants. The American Naturalist, 142(4): 718–724.
Bertness, M. D., & Silliman, B. R. 2008. Consumer Control of Salt Marshes Driven by Human Disturbance. Conservation Biology, 22(3): 618–623.
Bertness, M. D., & Yeh, S. M. 1994. Cooperative and Competitive Interactions in the Recruitment of Marsh Elders. Ecology, 75(8): 2416–2429.
Boon, J. D. 2012. Evidence of Sea Level Acceleration at U.S. and Canadian Tide Stations, Atlantic Coast, North America. Journal of Coastal Research, 1437–1445.
Bricker-Urso, S., Nixon, S. W., Cochran, J. K., Hirschberg, D. J., & Hunt, C. 1989. Accretion Rates and Sediment Accumulation in Rhode Island Salt Marshes. Estuaries, 12(4): 300–317.
Bromberg, K. D., & Bertness, M. D. 2005. Reconstructing New England salt marsh losses using historical maps. Estuaries, 28(6): 823–832.
Bruno, J. F., Stachowicz, J. J., & Bertness, M. D. 2003. Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution, 18(3): 119–125.
Buchsbaum, R., Valiela, I., & Teal, J. M. 1981. Grazing by Canada Geese and Related Aspects of the Chemistry of Salt Marsh Grasses. Colonial Waterbirds, 4(ArticleType: research-article / Full publication date: 1981 / Copyright © 1981 Waterbird Society): 126–131.
Cahoon, D. R., Day, Jr., J. W., & Reed, D. J. 1999. The influence of surface and shallow subsurface soil processes on wetland elevation: a synthesis. Current Topics in Wetland Biogeochemistry, 3: 72–88.
Cahoon, D. R., & Guntenspergen, G. R. 2010. Climate change, sea-level rise, and coastal wetlands. Our Changing Climate, 32(1).
Cahoon, D. R., Hensel, P. F., Spencer, T., Reed, D. J., McKee, K. L., & Saintilan, N. 2006. Coastal wetland vulnerability to relative sea-level rise: wetland elevation trends and process controls. Wetlands and natural resource management: 271–292. Springer.
Castillo, J. M., Rubio-Casal, A. E., & Figueroa, E. 2010. Cordgrass Biomass in Coastal Marshes. In M. N. B. Momba & F. Bux (Eds.), Biomass: 1–26. Sciyo, Croatia: InTech.
Charles, H., & Dukes, J. S. 2009. Effects of Warming and Altered Precipitation on Plant and Nutrient Dynamics of a New England Salt Marsh. Ecological Applications, 19(7): 1758–1773.
Connell, J. H., & Slatyer, R. O. 1977. Mechanisms of Succession in Natural Communities and Their Role in Community Stability and Organization. The American Naturalist, 111(982): 1119–1144.
Coverdale, T. C., Bertness, M. D., & Alteiri, A. H. 2013. Regional Ontogeny of New England Salt Marsh Die-Off. Conservation Biology, 27(5): 1041–1048.
Coverdale, T. C., Herrmann, N. C., Altieri, A. H., & Bertness, M. D. 2013. Latent impacts: the role of historical human activity in coastal habitat loss. Frontiers in Ecology and the Environment, 11(2): 69–74.
Cowardin, L. M., Carter, V., Golet, F. C., & LaRoe, E. T. 1979. Classification of Wetlands and Deepwater Habitats of the US. DIANE Publishing.
Craft, C., Clough, J., Ehman, J., Joye, S., Park, R., Pennings, S., et al. 2008. Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Frontiers in Ecology and the Environment, 7(2): 73–78.
Crain, C. M., Albertson, L. K., & Bertness, M. D. 2008. Secondary succession dynamics in estuarine marshes across landscape-scale salinity gradients. Ecology, 89(10): 2889–2899.
Crain, C. M., Gedan, K., Dionne, M., Silliman, B., & Grosholz, B. 2009. Tidal restrictions and mosquito ditching in New England marshes. University of California Press: Berkely, CA, USA.
Crain, C. M., Silliman, B. R., Bertness, S. L., & Bertness, M. D. 2004. Physical and biotic drivers of plant distribution across estuarine salinty gradients. Ecology, 85(9): 2539–2549.
Deegan, L. A., Johnson, D. S., Warren, R. S., Peterson, B. J., Fleeger, J. W., Fagherazzi, S., et al. 2012. Coastal eutrophication as a driver of salt marsh loss. Nature, 490(7420): 388–392.
Donnelly, J. P., & Bertness, M. D. 2001. Rapid shoreward encroachment of salt marsh cordgrass in response to accelerated sea-level rise. Proceedings of the National Academy of Sciences, 98(25): 14218–14223.
Donnelly, J. P., Bryant, S. S., Butler, J., Dowling, J., Fan, L., Hausmann, N., et al. 2001. 700 yr sedimentary record of intense hurricane landfalls in southern New England. Geological Society of America Bulletin, 113(6): 714–727.
Donnelly, J. P., Cleary, P., Newby, P., & Ettinger, R. 2004. Coupling instrumental and geological records of sea-level change: Evidence from southern New England of an increase in the rate of sea-level rise in the late 19th century. Geophysical Research Letters, 31(5): L05203.
Dreyer, G. D., Niering, W. A., & Ouellette, T. R. 1995. Tidal marshes of Long Island Sound: ecology, history and restoration. Connecticut College Arboretum.
Edinger, G. J., Evans, D. J., Gebauer, S., Howard, T. G., Hunt, D. M., & Olivero, A. M. 2002. Ecological Communities of New York State. Second Edition. http://www.dec.ny.gov/animals/29392.html, Albany, NY: New York Natural Heritage Program, New York Stae Department of Envioronmental Conservation.
Ellison, A. 1987. Effects of Competition, Disturbance, and Herbivory on Salicornia Europaea. Ecology, 68(3): 576–586.
Elmer, W. H., & Marra, R. E. 2011. New species of Fusarium associated with dieback of Spartina alterniflora in Atlantic salt marshes. Mycologia, 103(4): 806–819.
Emery, N. C., Ewanchuk, P. J., & Bertness, M. D. 2001. Competition and salt-marsh plant zonation: stress tolerators may be dominant competitors. Ecology, 82(9): 2471–2485.
Enser, R., Gregg, D., Sparks, C., August, P., Jordan, P., Coit, J., et al. 2011. Rhode Island Ecological Communities Classification. Kingston, RI.: Rhode Island Natural History Survey.
Erdle, S. Y., Davis, J. L., Sellner, K. G., & Chesapeake Research Consortium. 2008. Management, Policy, Science, and Engineering of Nonstructural Erosion Control in the Chesapeake Bay: Proceedings of the 2006 Living Shoreline Summit. Chesapeake Research Consortium.
Ewanchuk, P. J., & Bertness, M. D. 2003. Recovery of a Northern New England Salt Marsh Plant Community from Winter Icing. Oecologia, 136(4): 616–626.
Ewanchuk, P. J., & Bertness, M. D. 2004. Structure and Organization of a Northern New England Salt Marsh Plant Community. Journal of Ecology, 92(1): 72–85.
Fagherazzi, S., Kirwan, M. L., Mudd, S. M., Guntenspergen, G. R., Temmerman, S., D’Alpaos, A., et al. 2012. Numerical models of salt marsh evolution: Ecological, geomorphic, and climatic factors. Reviews of Geophysics, 50(1): RG1002.
Flowers, T. J., Hajibagheri, M. A., & Clipson, N. J. W. 1986. Halophytes. The Quarterly Review of Biology, 61(3): 313–337.
Friedrichs, C. T., & Perry, J. E. 2001. Tidal salt marsh morphodynamics: a synthesis. Journal of Coastal Research, 7–37.
Gawler, S. C., & Cutko, A. 2010. Natural landscapes of Maine: A guide to natural communities and ecosystems. Maine Natural Areas Program, Department of Conservation.
Gedan, K. B., Altieri, A. H., & Bertness, M. D. 2011. Uncertain future of New England salt marshes. Marine Ecology Progress Series, 434: 229–237.
Gedan, K. B., Silliman, B., & Bertness, M. 2009. Centuries of human-driven change in salt marsh ecosystems. Annual Review of Marine Science, 1: 117–141.
Gedan, K., & Bertness, M. 2010. How will warming affect the salt marsh foundation species Spartina patens and its ecological role? Oecologia, 164(2): 479–487.
Grime, J. P. 2006. Plant strategies, vegetation processes, and ecosystem properties. John Wiley & Sons.
Harrison, E. Z., & Bloom, A. L. 1977. Sedimentation rates on tidal salt marshes in Connecticut. Journal of Sedimentary Research, 47(4): 1484–1490.
Hartig, E., Gornitz, V., Kolker, A., Mushacke, F., & Fallon, D. 2002. Anthropogenic and climate-change impacts on salt marshes of Jamaica Bay, New York City. Wetlands, 22(1): 71–89.
Hartman, J. M. 1984. The role of wrack disturbance in the vegetation of a New England salt marsh. Ph,D. Thesis. University of Connecticut, Storrs, CT. 260 p.
Holdredge, C., Bertness, M. D., & Altieri, A. H. 2009. Role of Crab Herbivory in Die-Off of New England Salt Marshes. Conservation Biology, 23(3): 672–679.
Hoover, M., Civco, D., & Whelchel, A. 2010. The development of a salt marsh migration tool and its application in Long Island Sound. Presented at the ASPRS 2010 Anual Conference, San Diego, CA.
IPCC. 2013a. Climate Change 2013. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the intergovernment Panel on Climate Change. Cambridge, UK and New York, NY, USA: Cambridge University Press.
IPCC. 2013b. Climate Change 2013: The Physical Science Basis. Contibution of Working Group I to the Fifth Assessment Report of the Intergovermental Panel on Climate Change. http://www.ipcc.ch/report/ar5/, May 1, 2014.
Jefferies, R. L., Jano, A. P., & Abraham, K. F. 2006. A biotic agent promotes large-scale catastrophic change in the coastal marshes of Hudson Bay. Journal of Ecology, 94(1): 234–242.
Kirwan, M. L., Guntenspergen, G. R., D’Alpaos, A., Morris, J. T., Mudd, S. M., & Temmerman, S. 2010. Limits on the adaptability of coastal marshes to rising sea level. Geophysical Research Letters, 37(23).
Kirwan, M. L., & Megonigal, J. P. 2013. Tidal wetland stability in the face of human impacts and sea-level rise. Nature, 504(7478): 53–60.
Koch, F., & Gobler, C. 2009. The Effects of Tidal Export from Salt Marsh Ditches on Estuarine Water Quality and Plankton Communities. Estuaries and Coasts, 32(2): 261–275.
Levine, J. M., Brewer, J. S., & Bertness, M. D. 1998. Nutrients, Competition and Plant Zonation in a New England Salt Marsh. Journal of Ecology, 86(2): 285–292.
Linthurst, R. A. 1979. The Effect of Aeration on the Growth of Spartina alterniflora Loisel. American Journal of Botany, 66(6): 685–691.
Mcleod, E., Poulter, B., Hinkel, J., Reyes, E., & Salm, R. 2010. Sea-level rise impact models and environmental conservation: A review of models and their applications. Ocean & Coastal Management, 53(9): 507–517.
Metzler,, K. J., & Barrett, J. P. 2006. The Vegetation of Connecticut - A Preliminary Classification. Hartford, CT: Stae Geological and Natural History Survey of Connecticut, Department of Environmental Protection.
Miller, W. R., & Egler, F. E. 1950. Vegetation of the Wequetequock-Pawcatuck Tidal-Marshes, Connecticut. Ecological Monographs, 20(2): 143–172.
Morris, J. T., Sundareshwar, P. V., Nietch, C. T., Kjerfve, B., & Cahoon, D. R. 2002. esponces of coastal wetlands to rising sea level. Ecology, 83(10): 2869–2877.
Nichols, G. E. 1920. The Vegetation of Connecticut. VII. The Associations of Depositing Areas Along the Seacoast. Bulletin of the Torrey Botanical Club, 47(11): 511–548.
Niering, W. A., & Warren, R. S. 1980. Vegetation Patterns and Processes in New England Salt Marshes. BioScience, 30(5): 301–307.
Nixon, S. W. 1982. The ecology of New England high salt marshes: a community profile. National Coastal Ecosystems Team, Washington, DC (USA); Rhode Island Univ., Kingston, RI (USA). Graduate School of Oceanography.
NOAA/NOS/CO-OPS. 2014, January 29. Sea Level Trends - NOAA Tides & Currents. http://tidesandcurrents.noaa.gov/sltrends/, January 29, 2014.
Odum, W. E. 1988. Comparative Ecology of Tidal Freshwater and Salt Marshes. Annual Review of Ecology and Systematics, 19: 147–176.
Odum, W. E., Smith III, T. J., Hoover, J. K., & McIvor, C. C. 1984. Ecology of tidal freshwater marshes of the United States east coast: A community profile. Virginia Univ., Charlottesville (USA). Dept. of Environmental Sciences.
Pace, M. L., Cole, J. J., Carpenter, S. R., & Kitchell, J. F. 1999. Trophic cascades revealed in diverse ecosystems. Trends in Ecology & Evolution, 14(12): 483–488.
Potente, J. E. 2007. Geomorphic Alteration of Tidal Wetlands by Mosquito Control Agencies. Unpubl. Rpt. SUNY-Stoney Brook.
Power, M. E. 1992. Top-Down and Bottom-Up Forces in Food Webs: Do Plants Have Primacy. Ecology, 73(3): 733–746.
Redfield, A. C. 1972. Development of a New England Salt Marsh. Ecological Monographs, 42(2): 201–237.
Reed, D. J. 1995. The response of coastal marshes to sea-level rise: Survival or submergence? Earth Surface Processes and Landforms, 20(1): 39–48.
Rochlin, I., James-Pirri, M.-J., Adamowicz, S. C., Dempsey, M. E., Iwanejko, T., & Ninivaggi, D. V. 2012. The effects of Integrated Marsh Management (IMM) on salt marsh vegetation, nekton, and birds. Estuaries and coasts, 35(3): 727–742.
Rochlin, I., James-Pirri, M.-J., Adamowicz, S., Wolfe, R., Capotosto, P., Dempsey, M., et al. 2012. Integrated Marsh Management (IMM): a new perspective on mosquito control and best management practices for salt marsh restoration. Wetlands Ecology and Management, 20(3): 219–232.
Roman, C. T. 2012. Tidal marsh restoration: a synthesis of science and management. Island Press.
Roman, C. T., Niering, W. A., & Warren, R. S. 1984. Salt marsh vegetation change in response to tidal restriction. Environmental Management, 8(2): 141–149.
Rozsa, R. 1995. Human impacts on tidal wetlands: history and regulations. Tidal Marshes of Long Island Sound: Ecology, History, and Restoration, Connecticut College Arboretum Bull, (34).
Sala, N. M., Bertness, M. D., & Silliman, B. R. 2008. The dynamics of bottom–up and top–down control in a New England salt marsh. Oikos, 117(7): 1050–1056.
Sallenger, A. H., Doran, K. S., & Howd, P. A. 2012. Hotspot of accelerated sea-level rise on the Atlantic coast of North America. Nature Clim. Change, 2(12): 884–888.
Silliman, B. R., Grosholz, E. D., & Bertness, M. D. 2009. Human Impacts on Salt Marshes: a Global Perspective. Berkeley and Los Angeles, CA: niversity of California Press.
Smith, J. P., & Carullo, M. 2007. Survey of Potential Marsh Dieback Sites in Coastal Massachusetts. Unpubl.
Smith, S. M. 2009. Multi-decadal changes in salt marshes of Cape Cod, MA: photographic analyses of vegetation loss, species shifts, and geomorphic change. Northeastern Naturalist, 16(2): 183–208.
Smith, T. J., & Odum, W. E. 1981. The Effects of Grazing by Snow Geese on Coastal Salt Marshes. Ecology, 62(1): 98–106.
Sperduto, D. D., & Nichols, W. F. 2012. Natural Communities of New Hampshire Second Edition. Concord, NH: NH Natural Heritage Bureau.
Swain, P. C., & Kearsley, J. B. 2012. Classification of the Natural Communities of Massachusetts. Version 1.4. Westborough, MA: Natural Heritage & Endangered Species Program, Division of Fisheries & Wildlife.
Teal, J. M. 1986. The ecology of regularly flooded salt marshes of New England: A community profile. Woods Hole Oceanographic Institution, MA (USA).
Theve, M. C. 2013. Halinity in Tidal Soils of the Connecticut River. http://digital commons.uconn.edu/gs_theses/427, Storrs, CT: University of Connecticut.
Tiner, R. W. 2013. Tidal Wetlands Primer. University of Massachusetts Press.
Tiner, R. W., Huber, I. J., Nuerminger, T., & Marshall, E. 2006. Salt marsh trends in selected estuaries of Southwestern Connecticut. UMASS.
Tonjes, D. J. 2013. Impacts from ditching salt marshes in the mid-Atlantic and northeastern United States. Environmental Reviews, 21(2): 116–126.
Turner, R. E. 2011. Beneath the Salt Marsh Canopy: Loss of Soil Strength with Increasing Nutrient Loads. Estuaries and Coasts, 34(5): 1084–1093.
Turner, R. E., Howes, B. L., Teal, J. M., Milan, C. S., Swenson, E. M., & Goehringer-Toner, D. D. 2009. Salt marshes and eutrophication: An unsustainable outcome. Limnology and Oceanography, 54(5): 1634.
Tyrrell, M. C., Dionne, M., & Edgerly, J. A. 2008. Physical factors mediate effects of grazing by a non-indigenous snail species on saltmarsh cordgrass (Spartina alterniflora) in New England marshes. ICES Journal of Marine Science: Journal du Conseil, 65(5): 746–752.
UNESCO. 1981. Background papers and supporting data on the practical salinity scale 1978. Technical Papers in Marine Science, 37.
Valiela, I., & Rietsma, C. S. 1995. Disturbance of Salt Marsh Vegetation by Wrack Mats in Great Sippewissett Marsh. Oecologia, 102(1): 106–112.
Valiela, I., Teal, J. M., & Persson, N. Y. 1976. Production and dynamics of experimentally enriched salt marsh vegetation: belowground biomass. Limnology and Oceanography, 21(2): 245–252.
Van de Plassche, O. 1991. Late Holocene Sea-Level Fluctuations on the Shore of Connecticut Inferred from Transgressive and Regressive Overlap Boundaries in Salt-Marsh Deposits. Journal of Coastal Research, (ArticleType: research-article / Issue Title: SPECIAL ISSUE NO. 11. Quaternary Geology of Long Island Sound and Adjacent Coastal Areas: Walter S. Newman Memorial Volume / Full publication date: FALL 1991 / Copyright © 1991 Coastal Education & Research Foundation, Inc.): 159–179.
Varekamp, J. C., & Thomas, E. 1998. Climate change and the rise and fall of sea level over the millennium. Eos, Transactions American Geophysical Union, 79(6): 69–75.
Vincent, R., Burdick, D., & Dionne, M. 2013. Ditching and Ditch-Plugging in New England Salt Marshes: Effects on Plant Communities and Self-Maintenance. Estuaries and Coasts, 1–15.
Warren, R. S., Fell, P. E., Rozsa, R., Brawley, A. H., Orsted, A. C., Olson, E. T., et al. 2002. Salt Marsh Restoration in Connecticut: 20 Years of Science and Management. Restoration Ecology, 10(3): 497–513.
Weinstein, M., Teal, J., Balletto, J., & Strait, K. 2001. Restoration principles emerging from one of the world’s largest tidal marsh restoration projects. Wetlands Ecology and Management, 9(5): 387–407.
Weston, N. 2014. Declining Sediments and Rising Seas: an Unfortunate Convergence for Tidal Wetlands. Estuaries and Coasts, 37(1): 1–23.
Zhang, L., & Shao, H. 2013. Direct plant–plant facilitation in coastal wetlands: A review. Estuarine, Coastal and Shelf Science, 119: 1–6.
Contributors
Nels Barrett Ph.D.
Approval
Greg Schmidt, 10/04/2024
Acknowledgments
Marissa Theve and the tech team acknowledged for compiling the soils and MLRA information.
Nels Barrett, Ph.D. ESI NRCS Tolland
Marissa Theve SS NRCS Tolland
Donald Parizek SSOL NRCS Tolland
Jacob Isleib SS NRCS Tolland
Deb Surabian SSS Tolland
Paul Capatosto CTDEEP Wildlife
Roger Wolfe CTDEEP Wildlife
Rangeland health reference sheet
Interpreting Indicators of Rangeland Health is a qualitative assessment protocol used to determine ecosystem condition based on benchmark characteristics described in the Reference Sheet. A suite of 17 (or more) indicators are typically considered in an assessment. The ecological site(s) representative of an assessment location must be known prior to applying the protocol and must be verified based on soils and climate. Current plant community cannot be used to identify the ecological site.
| Author(s)/participant(s) | Nels Barrett, Ph.D. |
|---|---|
| Contact for lead author | |
| Date | 07/03/2014 |
| Approved by | Greg Schmidt |
| Approval date | |
| Composition (Indicators 10 and 12) based on | Foliar Cover |
Indicators
-
Number and extent of rills:
N/A -
Presence of water flow patterns:
Semidiuranal tidal exchange -
Number and height of erosional pedestals or terracettes:
N/A -
Bare ground from Ecological Site Description or other studies (rock, litter, lichen, moss, plant canopy are not bare ground):
Bare ground is typically less than 50% based upon spacing between individual plants, but can increase to 70% bare ground due to chronic disturbances like ice rafting/scouring and wave erosion, up to 100% bare ground due to dieback. -
Number of gullies and erosion associated with gullies:
N/A -
Extent of wind scoured, blowouts and/or depositional areas:
occasional wave / ice scouring -
Amount of litter movement (describe size and distance expected to travel):
Regularly flooding semidiurnal tides removes all litter. -
Soil surface (top few mm) resistance to erosion (stability values are averages - most sites will show a range of values):
Low marsh soil/substrate checked by grass rhizomes and bysall threads of ribbed mussels.
Exposed soil surface suceptable to erosion in proportion to the magnitude of the flood disturbance e.g., greatest in coastal storms. -
Soil surface structure and SOM content (include type of structure and A-horizon color and thickness):
Oe - 0-25 cm very dark gray (10YR 3/1), structureless and massive, 45% SOM -
Effect of community phase composition (relative proportion of different functional groups) and spatial distribution on infiltration and runoff:
N/A salt marsh soils are saturated, and flooded daily by tides -
Presence and thickness of compaction layer (usually none; describe soil profile features which may be mistaken for compaction on this site):
N/A -
Functional/Structural Groups (list in order of descending dominance by above-ground annual-production or live foliar cover using symbols: >>, >, = to indicate much greater than, greater than, and equal to):
Dominant:
grassesSub-dominant:
Other:
forbsAdditional:
-
Amount of plant mortality and decadence (include which functional groups are expected to show mortality or decadence):
Perennial grasses will naturally exhibit a minor amount (less than 5%) of scenscence each year. -
Average percent litter cover (%) and depth ( in):
Litter is removed by regular semi-diurnal tidal flooding. -
Expected annual annual-production (this is TOTAL above-ground annual-production, not just forage annual-production):
3500 to 12000 lbs/acre (4000 to 13500 kg/ha) -
Potential invasive (including noxious) species (native and non-native). List species which BOTH characterize degraded states and have the potential to become a dominant or co-dominant species on the ecological site if their future establishment and growth is not actively controlled by management interventions. Species that become dominant for only one to several years (e.g., short-term response to drought or wildfire) are not invasive plants. Note that unlike other indicators, we are describing what is NOT expected in the reference state for the ecological site:
N/A -
Perennial plant reproductive capability:
All plants expected to reproduce annually unless disrupted by catastophic events prior to reproductive phase.
Print Options
Sections
Font
Other
The Ecosystem Dynamics Interpretive Tool is an information system framework developed by the USDA-ARS Jornada Experimental Range, USDA Natural Resources Conservation Service, and New Mexico State University.
Click on box and path labels to scroll to the respective text.