Natural Resources
Conservation Service
Ecological site R026XY031NV
SILTY 8-10 P.Z.
Last updated: 4/10/2024
Accessed: 12/09/2025
General information
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
MLRA notes
Major Land Resource Area (MLRA): 026X–Carson Basin and Mountains
The area lies within western Nevada and eastern California, with about 69 percent being within Nevada, and 31 percent being within California. Almost all this area is in the Great Basin Section of the Basin and Range Province of the Intermontane Plateaus. Isolated north-south trending mountain ranges are separated by aggraded desert plains. The mountains are uplifted fault blocks with steep side slopes. Most of the valleys are drained by three major rivers flowing east across this MLRA. A narrow strip along the western border of the area is in the Sierra Nevada Section of the Cascade-Sierra Mountains Province of the Pacific Mountain System. The Sierra Nevada Mountains are primarily a large fault block that has been uplifted with a dominant tilt to the west. This structure leaves an impressive wall of mountains directly west of this area. This helps create a rain shadow affect to MLRA 26. Parts of this eastern face, but mostly just the foothills, mark the western boundary of this area. Elevations range from about 3,806 feet (1,160 meters) on the west shore of Pyramid Lake to 11,653 feet (3,552 meters) on the summit of Mount Patterson in the Sweetwater Mountains.
Valley areas are dominantly composed of Quaternary alluvial deposits with Quaternary playa or alluvial flat deposits often occupying the lowest valley bottoms in the internally drained valleys, and river deposited alluvium being dominant in externally drained valleys. Hills and mountains are dominantly Tertiary andesitic flows, breccias, ash flow tuffs, rhyolite tuffs or granodioritic rocks. Quaternary basalt flows are present in lesser amounts, and Jurassic and Triassic limestone and shale, and Precambrian limestone and dolomite are also present in very limited amounts. Also of limited extent are glacial till deposits along the east flank of the Sierra Nevada Mountains, the result of alpine glaciation.
The average annual precipitation in this area is 5 to 36 inches (125 to 915 millimeters), increasing with elevation. Most of the rainfall occurs as high-intensity, convective storms in spring and autumn. Precipitation is mostly snow in winter. Summers are dry. The average annual temperature is 37 to 54 degrees F (3 to 12 degrees C). The freeze-free period averages 115 days and ranges from 40 to 195 days, decreasing in length with elevation.
The dominant soil orders in this MLRA are Aridisols and Mollisols. The soils in the area dominantly have a mesic soil temperature regime, an aridic or xeric soil moisture regime, and mixed or smectitic mineralogy. They generally are well drained, are clayey or loamy and commonly skeletal, and are very shallow to moderately deep.
This area supports shrub-grass vegetation characterized by big sagebrush. Low sagebrush and Lahontan sagebrush occur on some soils. Antelope bitterbrush, squirreltail, desert needlegrass, Thurber needlegrass, and Indian ricegrass are important associated plants. Green ephedra, Sandberg bluegrass, Anderson peachbrush, and several forb species also are common. Juniper-pinyon woodland is typical on mountain slopes. Jeffrey pine, lodgepole pine, white fir, and manzanita grow on the highest mountain slopes. Shadscale is the typical plant in the drier parts of the area. Sedges, rushes, and moisture-loving grasses grow on the wettest parts of the wet flood plains and terraces. Basin wildrye, alkali sacaton, saltgrass, buffaloberry, black greasewood, and rubber rabbitbrush grow on the drier sites that have a high concentration of salts.
Some of the major wildlife species in this area are mule deer, coyote, beaver, muskrat, jackrabbit, cottontail, raptors, pheasant, chukar, blue grouse, mountain quail, and mourning dove. The species of fish in the area include trout and catfish. The Lahontan cutthroat trout in the Truckee River is a threatened and endangered species.
LRU notes
The Semiarid Fans and Basins LRU includes basins, alluvial fans and adjacent hill slopes immediately east of the Sierra Nevada mountain range and are affected by its climate or have its granitic substrate. Elevations range from 1355 to 1920 meters and slopes range from 0 to 30 percent, with a median value of 6 percent. Frost free days range from 121 to 170.
Ecological site concept
The Silty 8-10 P.Z. site occurs on lower piedmont slopes. Slopes range from 0 to 2 percent. Elevations are 5040 to 5060 feet. The soils are typically very deep and well drained. The surface layers are free of salt and sodium. Additional moisture is received on this site as overflow from adjacent ephemeral streams or as run-in from higher landscapes. Runoff is low and ponding occurs in some areas particularly following intense storms or low elevation snow melt. The dominant plants are winterfat (Kraschninnikovia lanata) and Indian ricegrass (Achnatherum hymenoides).
This site was seen once during fieldwork for the Disturbance Response Group project. It is limited in extent in MLRA 26 with only 3 map units. This site is primarily mapped on private lands and much of it has been converted to farmland. For this reason, much of this report is adapted from similar ecological sites in MLRA 28A and 28B. Edits to this model may be warranted.
Associated sites
| R026XY012NV |
DRY FLOODPLAIN 8-10 P.Z. |
|---|---|
| R026XY016NV |
LOAMY 8-10 P.Z. |
| R026XY047NV |
DROUGHTY CLAYPAN 8-10 P.Z. |
Table 1. Dominant plant species
| Tree |
Not specified |
|---|---|
| Shrub |
(1) Krascheninnikovia lanata |
| Herbaceous |
(1) Achnatherum hymenoides |
Physiographic features
The Silty 8-10 P.Z. site occurs on lower piedmont slopes. Slopes range from 0 to 2 percent. Elevations are 5040 to 5060 feet.
Table 2. Representative physiographic features
| Landforms |
(1)
Fan piedmont
|
|---|---|
| Flooding duration | Very brief (4 to 48 hours) |
| Flooding frequency | Rare |
| Ponding frequency | None |
| Elevation | 1,536 – 1,542 m |
| Slope | 0 – 2% |
| Aspect | Aspect is not a significant factor |
Climatic features
The climate associated with this site is semiarid, characterized by cool, moist winters and warm, dry summers. Average annual precipitation is 8 to 10 inches. Mean annual air temperature is 48 to 50 degrees F. The average growing season is about 60 to 115 days.
Nevada’s climate is predominantly arid, with large daily ranges of temperature, infrequent severe storms, heavy snowfall in the higher mountains, and great location variations with elevation. Three basic geographical factors largely influence Nevada’s climate: continentality, latitude, and elevation. Continentality is the most important factor. The strong continental effect is expressed in the form of both dryness and large temperature variations. Nevada lies on the eastern, lee side of the Sierra Nevada Range, a massive mountain barrier that markedly influences the climate of the State. The prevailing winds are from the west, and as the warm moist air from the Pacific Ocean ascend the western slopes of the Sierra Range, the air cools, condensation occurs and most of the moisture falls as precipitation. As the air descends the eastern slope, it is warmed by compression, and very little precipitation occurs. The effects of this mountain barrier are felt not only in the West but throughout the state, with the result that the lowlands of Nevada are largely desert or steppes. The temperature regime is also affected by the blocking of the inland-moving maritime air. Nevada sheltered from maritime winds, has a continental climate with well-developed seasons and the terrain responds quickly to changes in solar heating.
Nevada lies within the mid-latitude belt of prevailing westerly winds which occur most of the year. These winds bring frequent changes in weather during the late fall, winter and spring months, when most of the precipitation occurs. To the south of the mid-latitude westerlies, lies a zone of high pressure in subtropical latitudes, with a center over the Pacific Ocean. In the summer, this high-pressure belt shifts northward over the latitudes of Nevada, blocking storms from the ocean. The resulting weather is mostly clear and dry during the summer and early fall, with scattered thundershowers. The eastern portion of the state receives significant summer thunderstorms generated from monsoonal moisture pushed up from the Gulf of California, known as the North American monsoon. The monsoon system peaks in August and by October the monsoon high over the Western U.S. begins to weaken and the precipitation retreats southward towards the tropics (NOAA 2004).
Table 3. Representative climatic features
| Frost-free period (characteristic range) | |
|---|---|
| Freeze-free period (characteristic range) | |
| Precipitation total (characteristic range) | 203-254 mm |
| Frost-free period (average) | 87 days |
| Freeze-free period (average) | |
| Precipitation total (average) | 229 mm |
Figure 1. Monthly average minimum and maximum temperature
Figure 2. Annual precipitation pattern
Figure 3. Annual average temperature pattern
Influencing water features
Additional moisture is received on the Silty 8-10 P.Z. site as overflow from adjacent ephemeral streams or as run-in from higher landscapes. Runoff is low and ponding occurs in some areas particularly following intense storms or low elevation snow melt. Potential for sheet and rill erosion is slight, however, the soils have a potential for formation of gullies, especially in areas near shallow drainageways.
Soil features
The soils are typically very deep and well drained. They are formed in alluvium from mixed rock sources. The surface layers are free of salt and sodium. Permeability is moderate to slow with high available water capacity. Additional moisture is received on this site as overflow from adjacent ephemeral streams or as run-in from higher landscapes. Runoff is low and ponding occurs in some areas particularly following intense storms or low elevation snow melt. Potential for sheet and rill erosion is slight, however, these soils have a potential for formation of gullies, especially in areas near shallow drainageways. The soil moisture regime is aridic bordering on xeric and the soil temperature regime is mesic.
The representative soil series associated with this site is Turria, a fine-loamy, mixed, superactive, mesic Xeric Haplargids. An ochric epipedon occurs from the soil surface to 5 cm and an argillic horizon occurs from 5 to 30 cm.
Table 4. Representative soil features
| Parent material |
(1)
Alluvium
|
|---|---|
| Surface texture |
(1) Silty clay loam |
| Family particle size |
(1) Loamy |
| Drainage class | Well drained |
| Permeability class | Moderately slow |
| Soil depth | 183 – 213 cm |
| Surface fragment cover <=3" | 0% |
| Surface fragment cover >3" | 0% |
| Available water capacity (0-101.6cm) |
17.78 – 20.07 cm |
| Calcium carbonate equivalent (0-101.6cm) |
0% |
| Electrical conductivity (0-101.6cm) |
0 mmhos/cm |
| Sodium adsorption ratio (0-101.6cm) |
0 |
| Soil reaction (1:1 water) (0-101.6cm) |
6.1 – 7.8 |
| Subsurface fragment volume <=3" (Depth not specified) |
0% |
| Subsurface fragment volume >3" (Depth not specified) |
0% |
Ecological dynamics
An ecological site is the product of all the environmental factors responsible for its development and it has a set of key characteristics that influence a site’s resilience to disturbance and resistance to invasive species. Key characteristics include 1) climate (precipitation, temperature), 2) topography (aspect, slope, elevation, and landform), 3) hydrology (infiltration, runoff), 4) soils (depth, texture, structure, organic matter), 5) plant communities (functional groups, productivity), and 6) natural disturbance regime (fire, herbivory, etc.) (Caudle et al. 2013). Biotic factors that influence resilience include site productivity, species composition and structure, and population regulation and regeneration (Chambers et al. 2013).
Winterfat is a long-lived, drought tolerant, native shrub typically about 30 cm tall (Mozingo 1987). It has a woody base from which annual branchlets grow(Welsh et al. 1987). The most common variety is a low growing dwarf form (less than 38.1 cm), which is most often found on desert valley floors(Stevens et al. 1977). Total winter precipitation is a primary growth driver and lower than average spring precipitation can reverse the impact of plentiful winter precipitation. While summer rainfall has a limited impact, heavy August-September rain can cause a second flowering in winterfat (West and Gasto 1978).
Winterfat reproduces from seed and primarily pollinates via wind(Stevens et al. 1977). Seed production, especially in desert regions, is dependent on precipitation(West and Gasto 1978)with good seed years occurring when there is appreciable summer precipitation and little browsing (Stevens et al. 1977).Winterfat has multiple dispersal mechanisms: diaspores are shed in the fall or winter, dispersed by wind, rodent-cached, or carried on animals (Majerus 2003). Diaspores take advantage of available moisture, tolerating freezing conditions as they progress from imbibed seeds to germinants to nonwoody seedlings(Booth 1989). Under some circumstances, the degree of reproduction may be dependent on mature plant density (Freeman and Emlen 1995).
These communities often exhibit the formation of microbiotic crusts within the interspaces between shrubs. These crusts influence the soils on these sites and their ability to reduce erosion and increase infiltration; they may also alter the soil structure and possibly increase soil fertility (Fletcher and Martin 1948, Williams 1993). Finer textured soils such as silts tend to support more microbiotic cover than coarse texture soils (Anderson 1982). Disturbance such as hoof action from inappropriate grazing and cheatgrass (Bromus tectorum) invasion can reduce biotic crust integrity (Anderson 1982, Ponzetti et al. 2007) and ecological sites in this DRG have low to moderate resilience to disturbance and resistance to invasion. Drought and/or inappropriate grazing will initially favor shrubs but prolonged drought can cause a decrease in the winterfat, and other shrubs while bare ground increases. Squirreltail may maintain or also decline within the community. Repeated spring and early summer grazing will have an especially detrimental effect on winterfat. Cheatgrass and other non-native annual weeds increase with excessive grazing. Abusive grazing during the winter may lead to soil compaction and reduced infiltration. Prolonged abusive grazing during any season leads to abundant bare ground, desert pavement and active wind and water erosion. Repeated, frequent fire will promote cheatgrass dominance and elimination of the native plant community.
These sites frequently attract recreational use, primarily by off highway vehicles (OHV). Annual non-native species increase where surface soils have been disturbed. Five alternative stable states have been identified for this site.
Fire Ecology:
Winterfat tolerates environmental stress, extremes of temperature and precipitation, and competition from other perennials but not the disturbance of fire or overgrazing (Ogle et al. 2001). Fire is rare within these communities due to low fuel loads. There are conflicting reports in the literature about the response of winterfat to fire. In one of the first published descriptions, Dwyer and Pieper (1967) reported that winterfat sprouts vigorously after fire. This observation was frequently cited in subsequent literature, but recent observations have suggested that winterfat can be completely killed by fire (Pellant and Reichert 1984).The response is dependent on fire severity. Winterfat is able to sprout from buds near the base of the plant. However, if these buds are destroyed, winterfat will not sprout. Research has shown that winterfat seedling growth is depressed in growth by at least 90% when growing in the presence of cheatgrass (Bromus tectorum) (Hild et al. 2007).Repeated, frequent fires will increase the likelihood of conversion to a non-native, annual plant community with trace amounts of winterfat.
Fourwing saltbush is the most widely distributed shrubby saltbush in North America (Meyer 2003). It is highly variable across landscapes and even within populations (McArthur et al. 1983, Petersen et al. 1987). Its ability to sprout following fire may depend on the population and fire severity. A study by Parmenter (2008) showed 58 percent mortality rate of fourwing saltbush following fire in New Mexico, the surviving shrubs produced sprouts shortly after fire. Indian ricegrass is the dominant grass on this ecological site. It is a hardy, cool-season, densely tufted, native perennial bunchgrass that grows from 4 to 24 inches in height (Blaisdell and Holmgren 1984).
Indian ricegrass has been found to reestablish on burned sites through seed dispersed from adjacent unburned areas (Young 1983). Thus the presence of surviving, seed producing plants is necessary for reestablishment of Indian ricegrass. Grazing management following fire to promote seed production and establishment of seedlings is important.
Bottlebrush squirreltail, another cool-season, native perennial bunchgrass is common to this ecological site. Bottlebrush squirreltail is considered more fire tolerant than Indian ricegrass due to its small size, coarse stems, and sparse leafy material (Britton et al. 1990). Post-fire regeneration occurs from surviving root crowns and from on-and off-site seed sources.
Livestock/Wildlife Grazing Interpretations:
Winterfat is a valuable forage species with an average of 10 percent crude protein during winter when there are few nutritious options for livestock and wildlife (Welch 1989). However, excessive grazing throughout the west has negatively impacted survival of winterfat stands (Hilton 1941, Statler 1967, Stevens et al. 1977).Time of grazing is critical for winterfat with the active growing period being most critical (Romo 1995). Stevens et al. (1977)found that both vigor and reproduction of winterfat were reduced in Steptoe Valley, Nevada by improper season of use. Stevens et al. (1977)recommended no more than 25% utilization during periods of active growth and up to 75% utilization during dormant season use. Rasmussen and Brotherson (1986)found significantly greater foliar cover and density of winterfat in areas ungrazed for 26 years versus winter grazed areas in Utah. In exclosures protected from grazing for between 5 and 16 years, Rice and Westoby (1978)found that winterfat increased in foliar cover but not in density where it was dominant, and in both foliar cover and density in shadscale-perennial grass communities where it was not dominant.
In addition to grazing by cattle, winterfat is browsed by rabbits, antelope, and other wildlife species (Stevens et al. 1977, Ogle et al. 2001). Winterfat and perennial grasses average 80% of jackrabbits’ diet in southeastern Idaho, with shrubs being grazed in fall and winter particularly (Johnson and Anderson 1984). Pronghorn and rabbits browse stems, leaves, and seed stalks of winterfat year round, especially during periods of active growth(Stevens et al. 1977). Management of wildlife browse is difficult and browse may be harmful to winterfat reestablishment as seed production and regrowth are curtailed if grazing occurs as the plant begins to grow (Eckert 1954).
Spiny hopsage is palatable to livestock, especially sheep, during the spring and early summer (Phillips et al. 1996). However, the shrub goes to seed and loses its leaves in July and August so its usefulness in the fall and winter is limited (Sanderson and Stutz 1992). Two studies showed little to no utilization by sheep during the winter (Harrison and Thatcher 1970, Green et al. 1951). Some scientists are concerned about the longevity of the species. One study showed no change in cover or density when excluded from livestock and wildlife grazing for 10+ years (Rice and Westoby 1978), while another seldom observed seedling establishment (Daubenmire 1970). With poor recruitment rates, some are concerned that with repeated fires and overgrazing, local populations of spiny hopsage may be lost (Simmons and Rickard 2003).
Fourwing saltbush is one of the most important forage shrubs in arid sites. Its importance is due to its abundance, accessibility, size, large volume of forage, evergreen habit, high palatability and nutritive value. The palatability rates from fairly good to good for cattle, and as good for sheep and goats, deer usually relish it as a winter browse (Dayton, 1937). It has similar protein, fat, and carbohydrate levels as alfalfa (Medicago sativa) (Catlin, 1925). It is especially valuable as winter forage. It was noted in a study by Otsyina et al. (1982)that sheep readily grazed fourwing saltbush when introduced into a new pasture.
Heavy spring grazing has been found to sharply reduce the vigor of Indian ricegrass and decrease the stand (Cook and Child 1971).In eastern Idaho, productivity of Indian ricegrass was at least 10 times greater in undisturbed plots than in heavily grazed ones (Pearson 1965). Cook and Child (1971)found significant reduction in plant cover after 7 years of rest from heavy (90 percent) and moderate (60 percent) spring use. The seed crop may be reduced where grazing is heavy (Bich et al. 1995). Tolerance to grazing increases after May thus spring deferment may be necessary for stand enhancement(Pearson 1964, Cook and Child 1971);however, utilization of less than 60% is recommended.
Bottlebrush squirreltail has the ability to produce large numbers of highly germinable seeds, with relatively rapid germination (Young and Evans 1977) when exposed to the correct environmental cues. Early spring growth and ability to grow at low temperatures contribute to the persistence of bottlebrush squirreltail among cheatgrass dominated ranges (Hironaka and Tisdale 1973). Squirreltail generally increases in abundance when moderately grazed or protected (Hutchings and Stewart 1953).In addition, moderate trampling by livestock in big sagebrush rangelands of central Nevada enhanced bottlebrush squirreltail seedling emergence compared to untrampled conditions. Heavy trampling however was found to significantly reduce germination sites (Eckert et al. 1987). Squirreltail is more tolerant of grazing than Indian ricegrass but all bunchgrasses are sensitive to over utilization within the growing season.
Reduced bunchgrass vigor or density provides an opportunity for cheatgrass and other invasive species to occupy interspaces. This can lead to increased fire frequency and potentially an annual plant community.
Annual Invasive Grasses:
The species most likely to invade these sites is cheatgrass. Cheatgrass is a cool season annual grass that maintains an advantage over native plants in part because it is a prolific seed producer, can germinate in the autumn or spring, tolerates grazing, and increases with frequent fire (Klemmedson and Smith 1964, Miller et al. 1999).Cheatgrass originated from Eurasia and was first reported in North America in the late 1800s (Mack and Pyke 1983; Furbush 1953). Pellant and Hall (1994) found 3.3 million acres of public lands dominated by cheatgrass and suggested that another 76 million acres were susceptible to invasion by winter annuals including cheatgrass and medusahead.
Recent modeling and empirical work by Bradford and Lauenroth (2006) suggests that seasonal patterns of precipitation input and temperature are also key factors determining regional variation in the growth, seed production, and spread of invasive annual grasses. The phenomenon of cheatgrass “die-off” provides opportunities for restoration of perennial and native species(Baughman et al. 2016, Baughman et al. 2017). The causes of these events are not fully understood, but there is ongoing work to try to predict where they occur, in the hopes of aiding conservation planning (Weisberg et al. 2017, Brehm 2019).
Methods to control cheatgrass include herbicide, fire, targeted grazing, and seeding. Mapping potential or current invasion vectors is a management method designed to increase the cost effectiveness of control methods. Spraying with herbicide (Imazapic or Imazapic + glyphosate) and seeding with crested wheatgrass and Sandberg bluegrass has been found to be more successful at combating cheatgrass (and medusahead) than spraying alone (Sheley et al. 2012). Butler et al. (2011) tested four herbicides (Imazapic, Imazapic + glyphosate, rimsulfuron,and sulfometuron + Chlorsulfuron) for suppression of cheatgrass, medusahead and ventenata (North Africa grass, Ventenata dubia) within residual stands of native bunchgrass. Additionally, they tested the same four herbicides followed by seeding of six bunchgrasses (native and non-native) with varying success (Butler et al. 2011). Herbicide-only treatments appeared to remove competition for established bluebunch wheatgrass by providing 100 percent control of ventenata and medusahead and greater than 95 percent control of cheatgrass (Butler et al. 2011). Caution in using these results is advised, as only one year of data was reported.
In considering pre-emergent herbicide for invasive annual grass control, it is important to assess the soil for characteristics that may reduce effectiveness. Imazapic, for example, is less effective in soils with high contents of sand; on the other hand, clay soils allow for excessive leaching (Inoue et al. 2009). Imazapic may be minimally effective on calcareous soils because the chemical binds to particles of organic matter more readily at high pH (Inoue et al. 2009, Tu et al. 2001). Effects on non-target plants should also be considered. Imazapic is readily adsorbed through foliage and roots (Tu et al. 2001) and can have negative effects on desirable plants, however most established perennial grasses remain unaffected (Applestein et al. 2018). Vollmer and Vollmer (2008) tested the tolerance of mountain mahogany (Cercocarpus montanus), antelope bitterbrush, and multiple sagebrush species to three rates of Imazapic with and without methylated seed oil as a surfactant. Sagebrush, regardless of species or rate of application, was not affected. However, many environmental variables were not reported in this study and managers should install test plots before broad scale herbicide application is initiated. Grasses drill-seeded after imazapic application displayed improved establishment rates, indicating that careful seeding can lead to restoration success, at least for the species studied (Morris et al. 2007).
After a wildfire, there is opportunity to intervene with seeding to establish perennial plants that will compete with cheatgrass. To date, most seeding success has occurred with non-native wheatgrass species. Perennial grasses, especially crested wheatgrass, are able to suppress cheatgrass growth when mature (Blank et al. 2020). Where native bunchgrasses are missing from the site, revegetation of annual grass invaded rangelands has been shown to have a higher likelihood of success when using introduced perennial bunchgrasses such as crested wheatgrass (Clements et al. 2017, Davies et al. 2015).
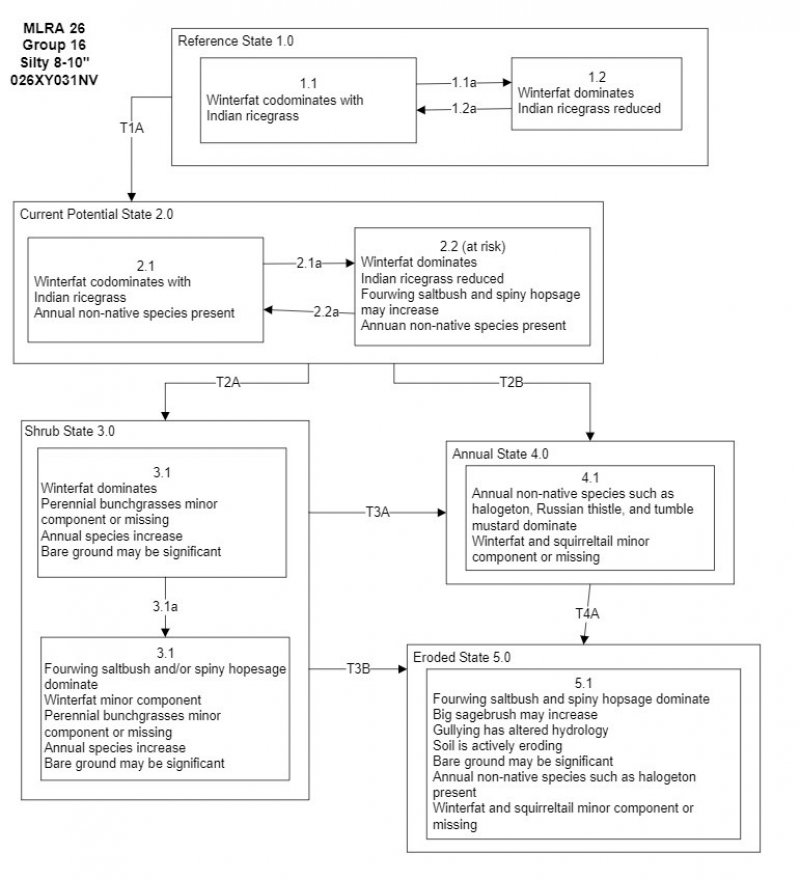
State and Transition Model Narrative
Reference State 1.0:
The Reference State 1.0 represents the natural range of variability under pristine conditions. This state has two community phases: one co-dominated by shrubs and grass, and the other dominated by shrubs. State dynamics are maintained by interactions between climatic patterns and disturbance regimes. Negative feedbacks enhance ecosystem resilience and contribute to the stability of the state. These include the presence of all structural and functional groups, low fine fuel loads, and retention of organic matter and nutrients. This site is very stable, with little variation in plant community composition. Plant community changes would be reflected in production in response to drought or abusive grazing. Wet years will increase grass production, while drought years will reduce production. Shrub production will also increase during wet years; however, recruitment of winterfat is episodic.
Community Phase 1.1:
This community is dominated by winterfat and Indian ricegrass. Fourwing saltbush is another important species on this site. Community phase changes are primarily a function of chronic drought. Fire is infrequent and patchy due to low fuel loads.
Community Phase Pathway 1.1a, from Phase 1.1 to 1.2: Long term drought and/or herbivory. Fires would also decrease vegetation on these sites but would be infrequent and patchy due to low fuel loads.
Community Phase 1.2:Drought will favor shrubs over perennial bunchgrasses. However, long-term drought will result in an overall decline in the plant community, regardless of functional group.
Community Phase Pathway 1.2a, from Phase 1.2 to 1.1: Time, lack of disturbance and recovery from drought would allow the vegetation to increase and bare ground would eventually decrease.
T1A: Transition from Reference State 1.0 to Current Potential State 2.0:Trigger: This transition is caused by the introduction of non-native annual plants, such as halogeton and cheatgrass.
Slow variables: Over time the annual non-native species will increase within the community.
Threshold: Any amount of introduced non-native species causes an immediate decrease in the resilience of the site. Annual non-native species cannot be easily removed from the system and have the potential to significantly alter disturbance regimes from their historic range of variation.
Current Potential State 2.0:
This state is similar to the Reference State 1.0. This state has the same two general community phases. Ecological function has not changed, however the resiliency of the state has been reduced by the presence of invasive weeds. Non-natives may increase in abundance but will not become dominant within this State. These non-natives can be highly flammable and can promote fire where historically fire had been infrequent. Negative feedbacks enhance ecosystem resilience and contribute to the stability of the state. These feedbacks include the presence of all structural and functional groups, low fine fuel loads, and retention of organic matter and nutrients. Positive feedbacks decrease ecosystem resilience and stability of the state. These include the non-natives’ high seed output, persistent seed bank, rapid growth rate, ability to cross pollinate, and adaptations for seed dispersal.
Community Phase 2.1:This community is dominated by winterfat and Indian ricegrass. Community phase changes are primarily a function of chronic drought. Fire is infrequent and patchy due to low fuel loads. Non-native annual species are present.
Community Phase Pathway 2.1a, from Phase 2.1 to 2.2:Long term drought will favor shrubs over perennial bunchgrasses. However, long-term drought will result in an overall decline in the plant community, regardless of functional group. Inappropriate grazing of winterfat will reduce this shrub and allow fourwing and spiny hopsage to increase.
Community Phase2.2: This community is dominated by winterfat. The perennial grass component is significantly reduced.
Community Phase Pathway 2.2a, from Phase 2.2 to 2.1: Release from long term drought and/or growing season grazing pressure allows recovery of bunchgrasses, winterfat, and bud sagebrush.
T2A: Transition from Current Potential State 2.0 to Shrub State 3.0:Trigger: Inappropriate, long-term grazing of perennial bunchgrasses during the growing season and/or long term drought will favor shrubs and initiate a transition to Community Phase 3.1.
Slow variables: Long term decrease in deep-rooted perennial grass density.
Threshold: Loss of deep-rooted perennial bunchgrasses changes nutrient cycling, nutrient redistribution, and reduces soil organic matter.
T2B: Transition from Current Potential State 2.0 to Annual State 4.0:
Trigger: Severe fire/ multiple fires, long term inappropriate grazing and/or soil disturbing treatments such as plowing. Slow variables: Increased production and cover of non-native annual species.
Threshold: Loss of deep-rooted perennial bunchgrasses and shrubs truncates, spatially and temporally, nutrient capture and cycling within the community. Increased, continuous fine fuels from annual non-native plants modify the fire regime by changing intensity, size and spatial variability of fires.
Shrub State 3.0:
This state consists of two community phases. The site in this phase has crossed a biotic threshold and site processes are being controlled by shrubs. Winterfat or sprouting shrubs like fourwing saltbush and spiny hopsage dominate the overstory. Indian ricegrass and other perennial bunchgrasses are reduced. Rabbitbrush may be a significant component. The shrub overstory dominates site resources such that soil water, nutrient capture, nutrient cycling and soil organic matter are temporally and spatially redistributed.
Community Phase 3.1:Perennial bunchgrasses, like Indian ricegrass are significantly reduced and the site is dominated by winterfat. Annual non-native species may be increasing. Bare ground has increased and there may be evidence of soil movement.
Community Phase Pathway 3.1a, from phase 3.1 to 3.2: Inappropriate grazing management that reduces winterfat viability gives spiny hopsage and fourwing saltbush a competitive edge. Winterfat is eventually pushed out of the system.
Community Phase 3.2: Spiny hopsage and fourwing saltbush dominate the site. Winterfat, Indian ricegrass, and other perennial bunchgrasses are minor components and may be missing. Annual non-native species may be present.
T3A: Transition from Shrub State 3.0 to Annual State 4.0:Trigger: Severe fire/multiple fires, long term inappropriate grazing, and/or soil disturbing treatments such as plowing.
Slow variables: Increased production and cover of non-native annual species.
Threshold: Increased, continuous fine fuels modify the fire regime by changing intensity, size and spatial variability of fires. Changes in plant community composition and spatial variability of vegetation due to the loss of perennial bunchgrasses and sagebrush truncate energy capture spatially and temporally thus impacting nutrient cycling and distribution.
T3B: Transition from Shrub State 3.0 to Eroded State 5.0:Trigger: Contiguous inappropriate grazing management and/or soil disturbance that concentrates runoff of water.
Slow variables: Increased bare ground.
Threshold: Headcutting and subsequent gullies alter the hydrology of the site. Loss of hydraulic connectivity alters the potential vegetation and truncates, spatially and temporally, nutrient capture and cycling within the community.
Annual State 4.0:
This state consists of one community phase. This community is characterized by the dominance of annual non-native species such as halogeton and cheatgrass. Rabbitbrush, fourwing saltbush, spiny hopsage, and other sprouting shrubs may dominate the overstory.
Community Phase 4.1:
This community is dominated by annual non-native species. Trace amounts of winterfat and other shrubs may be present, but are not contributing to site function. Bare ground may be abundant, especially during low precipitation years. Ecological dynamics are significantly altered in this state. Annual non-native species create a highly combustible fuel bed that shortens the fire return interval. Nutrient cycling is spatially and temporally truncated as annual plants contribute significantly less to deep soil carbon. Because this is a productive site, some deep-rooted perennial grasses may remain, even in the annual state. Without management, it is unlikely these plants will be able to recruit in the presence of dominant annual grasses. Soil erosion, soil temperature and wind are driving factors in site function.
T4A: Transition from Annual State 3.0 to Eroded State 5.0:
Trigger: Contiguous inappropriate grazing management and/or soil disturbance that concentrates runoff of water.
Slow variables: Increased bare ground.
Threshold: Headcutting and subsequent gullies alter the hydrology of the site. Loss of hydraulic connectivity alters the potential vegetation and truncates, spatially and temporally, nutrient capture and cycling within the community.
Eroded State 5.0:
This site consists of one community phase. Abiotic factors including soil redistribution and erosion, soil temperature, soil crusting and sealing are primary drivers of ecological condition within this state. Soil moisture, soil nutrients and soil organic matter distribution and cycling are severely altered due to degraded soil surface conditions and reduced seasonal flooding.
Community Phase 5.1: Big sagebrush, fourwing saltbush, and spiny hopsage dominate this phase. Winterfat and grasses are minor components and may be entirely missing from the site. Gullying and active soil erosion are occurring. Bare ground may be significant. Hydrology has been altered at this site due to significant soil loss. Annual non-native species such as halogeton and annual mustards may be present.
State and transition model
More interactive model formats are also available.
View Interactive Models
Click on state and transition labels to scroll to the respective text
Ecosystem states
State 1 submodel, plant communities
State 1
Reference State
Community 1.1
Reference Plant Community
The reference plant community is dominated by winterfat and Indian ricegrass. Potential vegetative composition is about 35% grasses, 5% forbs and 60% shrubs.
Figure 4. Annual production by plant type (representative values) or group (midpoint values)
Table 5. Annual production by plant type
| Plant type | Low (kg/hectare) |
Representative value (kg/hectare) |
High (kg/hectare) |
|---|---|---|---|
| Shrub/Vine | 202 | 269 | 404 |
| Grass/Grasslike | 118 | 157 | 235 |
| Forb | 17 | 22 | 34 |
| Total | 337 | 448 | 673 |
Additional community tables
Table 6. Community 1.1 plant community composition
| Group | Common name | Symbol | Scientific name | Annual production (kg/hectare) | Foliar cover (%) | |
|---|---|---|---|---|---|---|
|
Grass/Grasslike
|
||||||
| 1 | Primary Perennial Grasses | 108–179 | ||||
| Indian ricegrass | ACHY | Achnatherum hymenoides | 90–135 | – | ||
| squirreltail | ELEL5 | Elymus elymoides | 9–22 | – | ||
| Sandberg bluegrass | POSE | Poa secunda | 9–22 | – | ||
| 2 | Secondary Perennial Grasses | 9–22 | ||||
| needle and thread | HECOC8 | Hesperostipa comata ssp. comata | 2–9 | – | ||
|
Forb
|
||||||
| 3 | Perennial | 9–36 | ||||
| 4 | Annual | 1–13 | ||||
|
Shrub/Vine
|
||||||
| 5 | Primary Shrubs | 188–291 | ||||
| winterfat | KRLA2 | Krascheninnikovia lanata | 157–224 | – | ||
| fourwing saltbush | ATCA2 | Atriplex canescens | 22–45 | – | ||
| spiny hopsage | GRSP | Grayia spinosa | 9–22 | – | ||
| 6 | Secondary Shrubs | 4–18 | ||||
| yellow rabbitbrush | CHVI8 | Chrysothamnus viscidiflorus | 2–9 | – | ||
| littleleaf horsebrush | TEGL | Tetradymia glabrata | 2–9 | – | ||
Interpretations
Animal community
Livestock Interpretaions:
This site is suited to livestock grazing. Grazing management should be keyed to perennial grass and palatable shrub production. Indian ricegrass has good forage value for domestic sheep, cattle and horses. It supplies a source of green feed before most other native grasses have produced much new growth. Winterfat is an important forage plant for livestock, especially during winter when forage is scarce. Abusive grazing practices have reduced or eliminated winterfat on some areas even though it is fairly resistant to browsing. Effects depend on severity and season of grazing.
Stocking rates vary over time depending upon season of use, climate variations, site, and previous and current management goals. A safe starting stocking rate is an estimated stocking rate that is fine tuned by the client by adaptive management through the year and from year to year.
Wildlife Interpretations:
Winterfat is an important forage plant for wildlife, especially during winter when forage is scarce. Winterfat seeds are eaten by rodents and is a staple food for black-tailed jackrabbits. Mule deer and pronghorn antelope browse winterfat. Winterfat is used for cover by rodents and is potential nesting cover for upland game birds, especially when grasses grow up through its crown.
Hydrological functions
Runoff is low. Permeability is moderately slow.
Recreational uses
Aesthetic value is derived from the diverse floral and faunal composition and the colorful flowering of wild flowers and shrubs during the spring and early summer. This site offers rewarding opportunities to photographers and for nature study. This site is used for camping and hiking and has potential for upland and big game hunting
Other products
Indian ricegrass was traditionally eaten by some Native American peoples. The Paiutes used seed as a reserve food source.
Other information
Winterfat adapts well to most site conditions, and its extensive root system stabilizes soil. However, winterfat is intolerant of flooding, excess water, and acidic soils. Indian ricegrass is well-suited for surface erosion control and desert revegetation although it is not highly effective in controlling sand movement.
Supporting information
Type locality
| Location 1: Carson City County, NV | |
|---|---|
| General legal description | This site also occurs in Douglas, Lyon, Mineral, Storey, and Washoe Counties, Nevada. |
Other references
Anderson, D. C., K. T. Harper, and S. R. Rushforth. 1982. Recovery of Cryptogamic Soil Crusts from Grazing on Utah Winter Ranges. Journal of Range Management 35:355-359.
Banner, R. E. 1992. Vegetation Types of Utah. Rangelands 14:109-114.
Baughman, O. W., R. Burton, M.Williams, P. J. Weisberg, T. E. Dilts, and E. A. Leger. 2017. Cheatgrass die-offs: a unique restoration opportunity in northern Nevada. Rangelands 39(6):165-173.
Baughman, O. W., S. E. Meyer, Z. T. Aanderud, and E. A. Leger. 2016. Cheatgrass die-offs as an opportunity for restoration in the Great Basin, USA: Will local or commercial native plants succeed where exotic invaders fail? Journal of Arid Environments 124:193-204.
Bich, B. S., J. L. Butler, and C. A. Schmidt. 1995. Effects of Differential Livestock Use on Key Plant Species and Rodent Populations within Selected Oryzopsis hymenoides/Hilaria jamesii Communities of Glen Canyon National Recreation Area. The Southwestern Naturalist 40:281-287.
Blaisdell, J. P. and R. C. Holmgren. 1984. Managing Intermountain rangelands --salt-desert shrub ranges. Gen. Tech. Rep. INT-163. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station, Ogden, UT. p. 52
Blank, R. R., C. Clements, T. Morgan, D. Harmon, and F. Allen. 2020.Suppression of cheatgrass by perennial bunchgrasses. Rangeland Ecology & Management 73(6):766-771.
Booth, D. T. 1989. Seedbed ecology of winterfat: cations in diaspore bracts and their effects on germination and early plant growth. Journal of Range Management 42:178-182.
Bradford, J. B. and W. K. Lauenroth. 2006. Controls over invasion of Bromus tectorum: The importance of climate, soil, disturbance and seed availability. Journal of Vegetation Science 17(6): 693-704.
Brehm, J. R. 2019. Cheatgrass die-off in the Great Basin: A comparison of remote sensing detection methods and identification of environments favorable to die-off. M.S. Thesis. University of Nevada, Reno.
Britton, C. M., G. R. McPherson, and F. A. Sneva. 1990. Effects of burning and clipping on five bunchgrasses in eastern Oregon. Western North American Naturalist 50:115-120.
Butler, M., F. Brummer, J. Weber, and R. Simmons. 2011. Restoring Central Oregon Rangeland from Ventenata and Medusahead to a Sustainable Bunchgrass Environment –Warm Springs and Ashwood. Central Oregon Agriculture Research and Extension Center.
Catlin, C. N. 1925. Composition of Arizona Forages, with Comparative Data. College of Agriculture, University of Arizona, Tucson, AZ. 24 p.
Caudle, D., J. DiBenedetto, M. Karl, H.Sanchez, and C. Talbot. 2013. Interagency ecological site handbook for rangelands. Available at: http://jornada.nmsu.edu/sites/jornada.nmsu.edu/files/InteragencyEcolSiteHandbook.pdf. Accessed 4 October 2013.
Chambers, J. C. and B. E. Norton. 1993. Effects of grazing and drought on population dynamics of salt desert species on the Desert Experimental Range, Utah. Journal of Arid Environments 24:261-275.
Chambers, J., B. Bradley, C. Brown, C. D’Antonio, M. Germino, J. Grace, S. Hardegree, R. Miller, and D. Pyke. 2013. Resilience to Stress and Disturbance, and Resistance to Bromus tectorum L. Invasion in Cold Desert Shrublands of Western North America. Ecosystems 17:1-16.
Clements, C. D., D. N. Harmon, R. R. Blank, and M. Weltz. 2017. Improving seeding success on cheatgrass-infested rangelands in northern Nevada. Rangelands 39(6):174-181.
Cook, C. W. and R. D. Child. 1971. Recovery of desert plants in various states of vigor. Journal of Range Management 24:339-343.
Daubenmire, R. 1970. Steppe vegetation of Washington. Technical bulletin Washington Agriculture Experiment Station. 131 pp.
Daubenmire, R. 1970. Steppe Vegetation of Washington. Technical Bulletin 62. Washington Agricultural Experiment Station. 131 p.
Daubenmire, R. 1975. Plant succession on abandoned fields, and fire influences in a steppe area in southeastern Washington. Northwest Science 49:36-48.
Davies, K. W., C. S. Boyd, D. D. Johnson, A. M. Nafus, and M. D. Madsen. 2015. Success of seeding native compared with introduced perennial vegetation for revegetating medusahead-invaded sagebrush rangeland. Rangeland Ecology & Management 68(3):224-230.
Dayton, W. 1937. Range plant handbook. USDA, Forest Service. Bull(22).856 p.
Dwyer, D. D. and R. D. Pieper. 1967. Fire Effects on Blue Grama-Pinyon-Juniper Rangeland in New Mexico. Journal of Range Management 20:359-362.
Eckert, R. E., Jr. 1954. A Study of Competition Between Whitesage and Halogeton in Nevada. 7:223-225.
Eckert, R. E., Jr., F. F. Peterson, and F. L. Emmerich. 1987. A study of factors influencing secondary succession in the sagebrush [Artemisia spp. L.] type. Pages 149-168 in Proceedings: Seed and seedbed ecology of rangeland plants. U. S. Department of Agriculture, Agricultural Research Service, Tucson, A.Z.
Fletcher, J. E. and W. P. Martin. 1948. Some Effects of Algae and Molds in the Rain-Crust of Desert Soils. Ecology 29:95-100.
Freeman, D. C. and M. J. Emlen. 1995. Assessment of interspecific interactions in plant communities: an illustration from the cold desert saltbrush grassland of North America. Journal of Arid Environments 31:179-198.
Furbush, P. 1953. Control of Medusa-Head on California Ranges. Journal of Forestry 51(2): 118-121.
Green, L. R., L. A. Sharp, C. C.W., and L. E. Harris. 1951. Utilization of winter range forage by sheep. Journal of Range Management 4:233-241.
Harris, G. A. 1967. Some Competitive Relationships between Agropyron spicatum and Bromus tectorum. Ecological Monographs 37(2): 89-111.
Harrison, B. J., and A. P. Thatcher. 1970. Winter sheep grazing and forage preference in southwestern Wyoming. Journal of Range Management 23:109-111.
Hild, A. L., J. M. Muscha, and N. L. Shaw. 2007. Emergence and growth of four winterfat accessions in the presence of the exotic annual cheatgrass. In: R. E. Sosebee, D. B. Wester, C. M. Britton, E. D. McArthur, S. G. Kitchen [comps.]. Proceedings: Shrubland dynamics --fire and water RMRS-P-47. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Lubbock, TX. p. 147-152
Hilton, J. W. 1941. Effects of Certain Micro-Ecological Factors on the Germinability and Early Development of Eurotia Lanata. Northwest Science :86-92.
Hironaka, M. 1994. Medusahead: natural successor to the cheatgrass type in the northern Great Basin. Pages 89-91 in Proceedings of Ecology and Management of Annual Rangelands. Gen. Tech. Report INT-313. USDA Forest Service, Intermountain Research Station., Boise, ID.
Hironaka, M. and E. Tisdale. 1973. Growth and development of Sitanion hystrixand Poa sandbergii. Research Memorandum RM 72-24. U.S. International Biological Program, Desert Biome.
Hutchings, S. S. and G. Stewart. 1953. Increasing forage yields and sheep production on intermountain winter ranges. Circular No. 925. U.S. Department of Agriculture, Washington, D.C.
Inoue, M.H., Oliveira Jr, R.S., Constantin, J., Alonso, D.G. and Tormena, C.A., 2009. Bioavailability of diuron, imazapic and isoxaflutole in soils of contrasting textures. Journal of Environmental Science and Health Part B44(8):757-763.
Johnson, K. L. 1978. Wyoming shrublands: Proceedings, 7th Wyoming shrub ecology workshop. Page 58. University of Wyoming, Agricultural Extension Service, Rock Spring WY.
Johnson, R. D. and J. E. Anderson. 1984. Diets of black-tailed jack rabbits in relation to population density and vegetation. Journal of Range Management 37:79-83.
Klemmedson, J. O. and J. G. Smith. 1964. Cheatgrass (Bromus Tectorum L.). The botanical review 30(2): 226-262.
Mack, R. N. and D. Pyke. 1983. The demography of Bromus Tectorum: Variation in time and space. Journal of Ecology 71(1): 69-93.
Majerus, M. 2003. Winterfat Seeds (Krascheninnikovia lanata). Native Plants: 11 to 15.
Mangla, S., R. Sheley, and J. J. James. 2011. Field growth comparisons of invasive alien annual and native perennial grasses in monocultures. Journal of Arid Environments 75(2):206-210.
Monsen, S. B. 1992. The competitive influences of cheatgrass (Bromus tectorum) on site restoration. Pages 43-50 in Proceedings -Ecology, Management, and Restoration of Intermountain Annual Rangelands. General Technical Report INT-GTR-313. U.S.D.A Forest Service Intermountain Research Station, Boise, ID.
McArthur, E. D., R. Stevens, and A. C. Blauer. 1983. Growth performance comparisons among 18 accessions of fourwing saltbush [Atriplex canescens] at two sites in Central Utah. Journal of Range Management 36:78-81.
Meyer, S. E. 2003. Atriplex L. saltbush. Pages 283-289 in F. T. Bonner, editor. Woody plant seed manual. Agriculture Handbook 727. U.S. Department of Agriculture, Forest Service, Washington D.C.
Morris, C., T. A. Monaco, and C. W. Rigby. 2009. Variable impacts of imazapic rate on downy brome (Bromus tectorum) and seeded species in two rangeland communities. Invasive Plant Science and Management 2(2):110-119.
Mozingo, H. N. 1987. Shrubs of the Great Basin: A natural history. In: H. N. Mozingo, [ed.]. Shrubs of the Great Basin. University of Nevada Press, Reno NV. p. 67-72
Ogle, D. G., L. John, and L. Holzworth. 2001. Plant guide management and use of winterfat. USDA-NRCS, Boise, ID.
Otsyina, R., C. M. McKell, and E. Gordon Van. 1982. Use of Range Shrubs to Meet Nutrient Requirements of Sheep Grazing on Crested Wheatgrass during Fall and Early Winter. Journal of Range Management 35:751-753.
Parmenter, R. R. 2008. Long-term effects of a summer fire on desert grassland plant demographics in New Mexico. Rangeland Ecology & Management 61:156-168.
Pearson, L. 1964. Effect of harvest date on recovery of range grasses and shrubs. Agronomy Journal 56:80-82.
Pearson, L. C. 1965. Primary Production in Grazed and Ungrazed Desert Communities of Eastern Idaho. Ecology 46:278-285.
Pellant, M. and C. Hall. 1994. Distribution of two exotic grasses in intermountain rangelands: status in 1992, USDA Forest Service Gen. Tech Report INT-GTR-313S: 109-112.
Pellant, M. and L. Reichert. 1984. Management and rehabilitation of a burned winterfat community in southwestern Idaho. In McArthur, HC Stutz, and others [comps.], Proceeding of the symposium on the biology of Atriplex and related chenopods. United States Department of Agriculture, Forest Service General Technical Report INT-172. p. 281-285
Petersen, J. L., D. N. Ueckert, R. L. Potter, and J. E. Huston. 1987. Ecotypic variation in selected fourwing saltbush populations in Western Texas. Journal of Range Management 40:361-366.
Phillips, R. L., N. K. McDougald, and J. Sullins. 1996. Plant preference of sheep grazing the Mojave desert. Rangelands 18:141-144.
Ponzetti, J. M., B. McCune, and D. A. Pyke. 2007. Biotic Soil Crusts in Relation to Topography, Cheatgrass and Fire in the Columbia Basin, Washington. The Bryologist 110:706-722.
Rasmussen, L. L. and J. D. Brotherson. 1986. Response of Winterfat (Ceratoides lanata) Communities to Release from Grazing Pressure. Great Basin Naturalist 46:148-156.
Rice, B. and M. Westoby. 1978. Vegetative Responses of Some Great Basin Shrub Communities Protected against Jackrabbits or Domestic Stock. Journal of Range Management 31:28-34.
Rice, B., and M. Westoby. 1978. Vegetative responses of some Great Basin shrub communities protected against jackrabbits or domestic stock. Journal of Range Management 31:28-34.
Romo, J. T., Robert E. Redmann, Brendan L. Kowalenko and Andrew R. Nicholson. 1995. Growth of winterfat following defoliation in Northern Mixed Prairie of Saskatchewan. Journal of Range Management 48:240-245.
Sanderson, S. C., and H. C. Stutz. 1992, May 18-22. Woody chenopods useful for rangeland reclamation in western North America. Pages 374-378 in Proceedings: Ecology and Management of Annual Rangelands. Gen. Tech. Rep. INT-GTR-313. U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Boise,ID.
Sheley, R. L., E. A. Vasquez, A. Chamberlain, and B. S. Smith. 2012. Landscape-scale rehabilitation of medusahead (Taeniatherum caput-medusae) dominated sagebrush steppe. Invasive Plant Science and Management 5(4):436-442.
Simmons, S. A., and W. H. Rickard. 2003. Fire effects on spiny hopsage in south central Washington. Western North American Naturalist 63:524-528.Statler, G. D. 1967. Technical Note: Eurotia lanata Establishment Trials. Journal of Range Management 20:253-255.
Stevens, R., B. C. Giunta, K. Jorgensen, and A. P. Plummer. 1977. Winterfat (Ceratoides lanata). Federal Aid Project W-82-R, Utah State Division of Wildlife Resources, Utah.
Stubbendieck, J. L., S. L. Hatch, and C. H. Butterfield. 1992. North American range plants. University of Nebraska Press, Lincoln, NE.
Tisdale, E. W. and M. Hironaka. 1981. The sagebrush-grass region: A review of the ecological literature. University of Idaho, Forest, Wildlife and Range Experiment Station.
Tu, M., C. Hurd, and J. M. Randall. 2001. Weed control methods handbook: tools & techniques for use in natural areas. The Nature Conservancy, Wildland Invasive Species Team. 220 p.
Vollmer, J. L. and J. G. Vollmer (2008). Controlling cheatgrass in winter range to restore habitat and endemic fire Proceedings-Shrublands under fire: disturbance and recovery in a changing world. RMRS-P-52., Cedar City, UT, Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station.
Weisberg, P. J., T. E. Dilts, O. W. Baughman, S. E. Meyer, E. A. Leger, K. J. Van Gunst, and L. Cleeves. 2017. Development of remote sensing indicators for mapping episodic die-off of an invasive annual grass (Bromus tectorum) from the Landsat archive. Ecological Indicators 79:173-181.
Welch, B. L. 1989. Nutritive value of shrubs. In McKell, C.M. [ed.]. Academic Press, Inc. , San Diego, CA.
Welsh, S. L., N. D. Atwood, S. Goodrich, and L. C. Higgins. 1987. A Utah flora. The Great Basin Naturalist Memoir No. 9. Brigham Young University, Provo, Utah.
West, N. E. 1994. Effects of fire on salt-desert shrub rangelands. In: S. B. Monsen, S. G. Kitchen [eds.] Proceedings--Ecology and Management of Annual Rangelands Gen. Tech. Rep. INT-GTR-313. USDA, Forest Service, Intermountain Research Station, Boise, ID. p. 71-74
West, N. E. and J. Gasto. 1978. Phenology of the Aerial Portions of Shadscale and Winterfat in Curlew Valley, Utah. Journal of Range Management 31:43-45.Williams, J. D. 1993. Influence of microphytic crusts on selected soil physical and hydrologic properties in the Hartnet Draw Capital Reef National Park Utah. Dissertation. Range Science. Utah State University. Paper 2054. Available at: http://digitalcommons.usu.edu/etd/2054
Young, J. A. and R. A. Evans. 1977. Squirreltail Seed Germination. Journal of Range Management 30:33-36.
Young, R. P. 1983. Fire as a vegetation management tool in rangelands of the intermountain region. In S.B. Monsen, N. Shaw [eds.] Proceedings: Managing intermountain rangelands -improvement of range and wildlife habitats Gen. Tech. Rep. INT-GTR-157. U.S. Department of Agriculture, Forest Service. P. 18-31.
Contributors
DK/FR
Rangeland health reference sheet
Interpreting Indicators of Rangeland Health is a qualitative assessment protocol used to determine ecosystem condition based on benchmark characteristics described in the Reference Sheet. A suite of 17 (or more) indicators are typically considered in an assessment. The ecological site(s) representative of an assessment location must be known prior to applying the protocol and must be verified based on soils and climate. Current plant community cannot be used to identify the ecological site.
| Author(s)/participant(s) | |
|---|---|
| Contact for lead author | |
| Date | 12/09/2025 |
| Approved by | Kendra Moseley |
| Approval date | |
| Composition (Indicators 10 and 12) based on | Annual Production |
Indicators
-
Number and extent of rills:
-
Presence of water flow patterns:
-
Number and height of erosional pedestals or terracettes:
-
Bare ground from Ecological Site Description or other studies (rock, litter, lichen, moss, plant canopy are not bare ground):
-
Number of gullies and erosion associated with gullies:
-
Extent of wind scoured, blowouts and/or depositional areas:
-
Amount of litter movement (describe size and distance expected to travel):
-
Soil surface (top few mm) resistance to erosion (stability values are averages - most sites will show a range of values):
-
Soil surface structure and SOM content (include type of structure and A-horizon color and thickness):
-
Effect of community phase composition (relative proportion of different functional groups) and spatial distribution on infiltration and runoff:
-
Presence and thickness of compaction layer (usually none; describe soil profile features which may be mistaken for compaction on this site):
-
Functional/Structural Groups (list in order of descending dominance by above-ground annual-production or live foliar cover using symbols: >>, >, = to indicate much greater than, greater than, and equal to):
Dominant:
Sub-dominant:
Other:
Additional:
-
Amount of plant mortality and decadence (include which functional groups are expected to show mortality or decadence):
-
Average percent litter cover (%) and depth ( in):
-
Expected annual annual-production (this is TOTAL above-ground annual-production, not just forage annual-production):
-
Potential invasive (including noxious) species (native and non-native). List species which BOTH characterize degraded states and have the potential to become a dominant or co-dominant species on the ecological site if their future establishment and growth is not actively controlled by management interventions. Species that become dominant for only one to several years (e.g., short-term response to drought or wildfire) are not invasive plants. Note that unlike other indicators, we are describing what is NOT expected in the reference state for the ecological site:
-
Perennial plant reproductive capability:
Print Options
Sections
Font
Other
The Ecosystem Dynamics Interpretive Tool is an information system framework developed by the USDA-ARS Jornada Experimental Range, USDA Natural Resources Conservation Service, and New Mexico State University.
Click on box and path labels to scroll to the respective text.