Ecological site group F023XY919NV
Plateau Rims 12-16" PZ Western Juniper with Low Sagebrush and Idaho Fescue
Last updated: 06/03/2024
Accessed: 02/21/2026
Ecological site group description
Key Characteristics
- Site does not pond or flood
- Landform other than dunes
- Surface soils are not clayey
- Sites are tree dominated
- Elevations < 7000'
- Soils Clayey at PCS
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
Physiography
This group is on mountain slopes and plateau rims at elevations of 5,500 to 7,000 feet. Slopes are 5 to 15 percent.
Climate
The climate is classified as Cold Semi-Arid in the Koppen Classification System.
The area receives 12 to 16 inches of annual precipitation as snow in the winter and rain in spring and fall. Summers are generally dry.
The frost-free period is 70 to 100 days. The mean annual air temperature is 42 to 46 °F.
Soil features
The soils in this group are clayey and very shallow. They classify as Mollisols. The soil temperature regime is a mix of mesic and frigid. Representative soil series of this ecological site group include Bidrim and Crocan.
Vegetation dynamics
Ecological Dynamics and Disturbance Response:
An ecological site is the product of all the environmental factors responsible for its development. Each site has a set of key characteristics that influence its resilience to disturbance and resistance to invasives. According to Caudle et al. (2013), key characteristics include:
1. Climate factors such as precipitation and temperature.
2. Topographic characteristics such as aspect, slope, elevation, and landform.
3. Hydrologic processes such as infiltration and runoff.
4. Soil characteristics such as depth, texture, structure, and organic matter.
5. Plant communities and their functional groups and productivity.
6. Natural disturbance (fire, herbivory, etc.) regime.
Biotic factors that influence resilience include site productivity, species composition and structure, and population regulation and regeneration (Chambers et al., 2013).
Western Juniper:
In the past 140 years, western juniper (Juniperus occidentalis) expanded within its geographic range at unprecedented rates compared to any other time during the Holocene (Miller et al., 2005). Density of western juniper has increased since the middle of the 19th century (Tausch, 1999; Miller & Tausch, 2001). Western juniper woodlands in eastern Oregon with more than 10 percent canopy cover increased from 456,000 acres in 1936 to 2.2 million acres in 1988 (Gedney et al., 1999; Miller et al., 2005; Rowland et al., 2011). Causes for expansion of western juniper into sagebrush ecosystems include changes in the wildfire return interval, historic livestock grazing, and climate influences (Bunting, 1994; Soulé et al., 2003; Tausch, 1999; Miller et al., 2005). Mean fire return intervals prior to European settlement in mountain big sagebrush (Artemisia tridentata ssp. vaseyana) ecosystems were 15 to 25 years (Burkhardt & Tisdale, 1976; Young & Evans, 1981; Miller & Rose, 1999; Ansley et al., 2000), frequent enought to inhibit the encroachment of western juniper into these big sagebrush cover types (Miller & Tausch, 2001). Thus, trees were isolated to fire-safe areas such as rocky outcroppings and areas with low productivity.
Juniper growth is dependent mostly upon soil moisture stored from winter precipitation, mainly snow. Much of the summer precipitation is ineffective because it is lost either through runoff after summer convection storms or through evaporation and interception (Tueller & Clark, 1975). Juniper is highly resistant to drought, which is common in the Great Basin. Taproots of juniper have a relatively rapid rate of root elongation and are thus able to persist until precipitation conditions are more favorable (Emerson, 1932).
Infilling by younger trees increases tree canopy cover, causing a decrease in understory plant like sagebrush (Bates et al., 2000; Miller et al., 2000; Johnsen, 1962; Azuma et al., 2005). Furthermore, infilling shifts stand level biomass from ground fuels to canopy fuels, which has the potential to significantly impact fire behavior. The more tree-dominated juniper woodlands become, the less likely they are to burn under moderate conditions, resulting in infrequent, high-intensity fires (Gruell, 1999; Miller et al., 2008; Tausch, 1999). Additionally, as the understory vegetation declines in vigor, the ability of native perennial plants to recover after fire decreases (Urza et al., 2017). The increase in bare ground allows for the invasion of non-native annual species, such as cheatgrass (Bromus tectorum), and in conjunction with intensive wildfire the pontential for conversion to annual exotics is a serious threat (Tausch, 1999; Miller et al., 2008).
Specific successional pathways after disturbance in juniper stands depend on several variables: plant species present at the time of disturbance and their individual responses to disturbance, past management, type and size of disturbance, available seed sources in the soil or adjacent areas, and site and climatic conditions throughout the successional process.
Insects and diseases of western juniper are not well understood or studied (Eddleman et al., 1994). A fungus called Juniper Pocket Rot (Pyrofomes demidoffi), also known as white trunk rot (Eddleman et al., 1994; Durham, 2014), can kill Utah (Juniperus osteosperma) and western juniper. Pocket rot enters the tree through and wound or opening that exposes the heartwood. In an advanced stage, this fungus can cause high mortality (Durham, 2014). Dwarf mistletoe (Arceuthobium spp.) is a parasitic plant that may affect Utah juniper. Without treatment or pruning, it may kill the tree 10 to 15 years after infection. Seedlings and saplings are most susceptible to the parasite (Christopherson, 2014). Other diseases and pests that affect juniper include:
1. Witches' broom (Gymnosporangium sp.): Girdles and kills branches.
2. Leaf rust (Gymnosporangium sp.): Affects leaves and young branches.
3. Juniper blight (Phomopsis sp.)
4. Flat-head borers (Chrysobothris sp.): Attack the wood (Tueller & Clark, 1975).
5. Long-horned beetles (Methia juniperi, Styloxus bicolor), round-head borers (Callidium spp.): Girdle branches and can kill branches or entire trees (Tueller & Clark, 1975).
Understory Dynamics:
The understory of the ecological sites in this group is dominated by deep-rooted, cool-season, perennial bunchgrasses and long-lived shrubs (at least 50 years old) with high root to shoot ratios. The dominant shrubs usually root to the full depth of the winter-spring soil moisture recharge, which ranges from 1.0 to over 3.0 meters (Dobrowolski et al., 1990). Root length of mature sagebrush plants was measured to a depth of 2 meters in alluvial soils in Utah (Richards & Caldwell, 1987). However, community types with low sagebrush (Artemisia arbuscula) as the dominant shrub were found to have soil depths—and thus available rooting depths—of 71 to 81 centimeters in a study in northeast Nevada (Jensen, 1990). These shrubs have a flexible generalized root system with development of both deep taproots and laterals near the surface (Comstock & Ehleringer, 1992)
In the Great Basin, most annual precipitation is received during the winter and early spring. This continental semiarid climate regime favors growth and development of deep-rooted shrubs and herbaceous cool-season plants using the C3 photosynthetic pathway (Comstock & Ehleringer, 1992). Winter precipitation and slow melting of snow results in deeper percolation of moisture into the soil profile. Herbaceous plants, more shallow-rooted than shrubs, grow earlier in the growing season and thrive on spring rains, while the deeper-rooted shrubs lag in phenological development because they draw from deeply infiltrating moisture from snowmelt the previous winter. Periodic drought regularly influences sagebrush ecosystems, and the duration and severity of drought have increased throught the 20th century in much of the Intermountain West. Major shifts away from historical precipitation patterns have the greatest potential to alter ecosystem function and productivity. Species composition and productivity can be altered by the timing of precipitation and water availability within the soil profile (Bates et al., 2006).
Low sagebrush is fairly drought tolerant but also tolerates perched water tables during some portion of the growing season. Low sagebrush is also susceptible to the sagebrush defoliator known as the Aroga moth (Aroga websteri). While the Aroga moth can partially or entirely kill individual plants or entire stands of big sagebrush (Artemisia tridentata) (Furniss & Barr, 1975), research is inconclusive of the damage sustained by low sagebrush populations.
The perennial bunchgrasses generally have somewhat shallower root systems than the shrubs, but root densities are often as high as or higher than those of shrubs in the upper 50 centimeters but taper off more rapidly than shrubs. General differences in root depth distributions between grasses and shrubs result in resource partitioning in the understory.
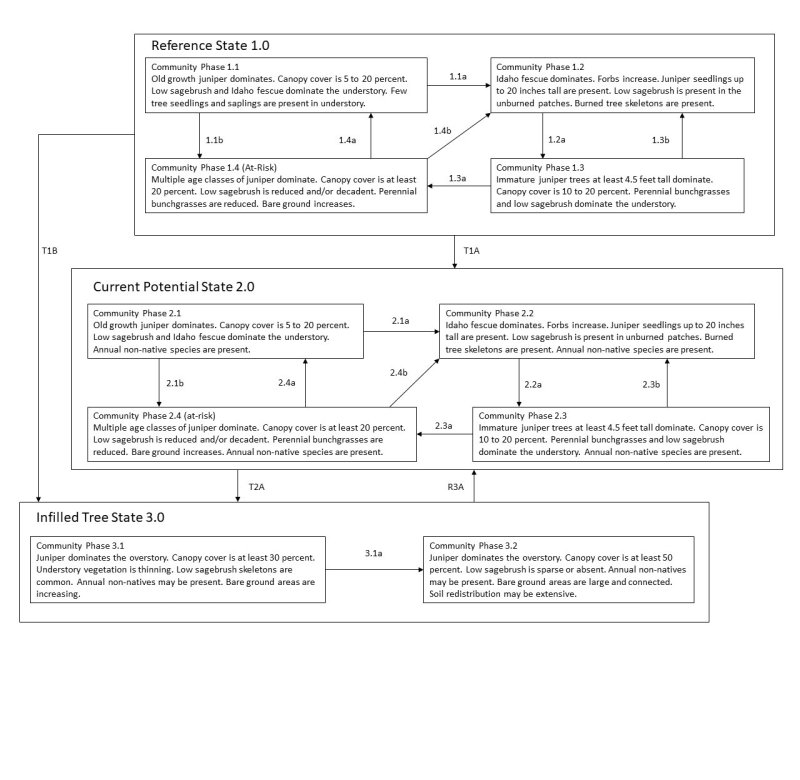
The ecological sites of this group have low to moderate resilience to disturbance and resistance to invasion. Resilience increases with higher elevation, northerly aspect, precipitation, and nutrient availability. Three possible states have been identified for this group.
Fire Ecology:
Large fires are rare on these sites. Lightning-ignited fires are common but typically do not affect more than a few individual trees (Miller & Rose, 1999). Western juniper is intolerant of fire and historically survived in areas with minimal understory vegetation, due primarily to soil characteristics (Vasek & Thorne, 1977; West, 1984; Miller & Rose, 1995). Therefore, the sites may not have carried fire, and when fire did occur it was low-intensity. With the increased suppression of wilfire and introduction of livestock grazing, which reduces ground fuels and understory competition, regeneration and establishment of western juniper has expanded into sites previously dominated by big sagebrush (Burns & Honkala, 1990). The expansion of western juniper is well documented. In the area surrounding Steens Mountain in southeastern Oregon, the expansion of western juniper coincides with Euro-American settlement. Probable causes of expansion include climate, altered fire frequencies, and grazing of flammable ground fuels (Miller & Rose, 1995). Fire resistance depends on age of the tree: seedlings, sapling, and poles are highly vulnerable to fire. Mature trees have some resistance to fire due to lack of fuels near the trunk, relatively thick bark, and foliage which is high above the ground (Burns & Honkala, 1990).
Literature is spare regarding historical fire return intervals in low sagebrush ecosystems. However, Miller and Rose (1999) did a study in an Oregon low sagebrush and juniper site and identified only two extensive fires between 1700 and 1880. Both fire events were preceded by at least 2 years of above average growth on the trees.
Low sagebrush is killed by fire and does not sprout (Tisdale & Hironaka, 1981). Establishment after fire is from seed, generally blown in and not from the seed bank (Bradley et al., 1992). Fire risk is greatest following a wet, productive year when there is greater production of fine fuels (Beardall & Sylvester, 1976). Fire return intervals have been estimated at 100 to 200 years in sites dominated by black sagebrush (Artemisia nova) (Kitchen & McArthur, 2007). Fire return intervals likely are similar in the low sagebrush ecosystem. However, historical fires were probably patchy due to the low productivity of these sites (Kitchen & McArthur, 2007). Fine fuel loads generally average 100 to 400 pounds per acre (110 to 450 kilograms per hectare) but are occasionally as high as 600 pounds per acre (680 kilograms per hectare) in low sagebrush habitat types (Bradley et al., 1992). Recovery time of low sagebrush following fire is variable (Young, 1983). After fire, if regeneration conditions are favorable, low sagebrush recovers in 2 to 5 years (Young, 1983). On harsh sites where cover is low to begin with and/or erosion occurs after fire, recovery may require more than 10 years (Young, 1983). Slow regeneration may subsequently worsen erosion (Blaisdell et al., 1982).
Antelope bitterbrush (Purshia tridentata) can be found on these ecological sites and is moderately fire tolerant (McConnell & Smith, 1977). It regenerates from seed and resprouting (Blaisdell & Mueggler, 1956; McArthur et al., 1983). However, sprouting ability is highly variable and is attributed to genetics, plant age, phenology, soil moisture, soil texture, and fire severity (Blaisdell & Mueggler, 1956; Blaisdell et al., 1982; Clark et al., 1982; Cook et al., 1994). Bitterbrush sprouts from a region on the stem approximately 1.5 inches above and below the soil surface; the plant rarely sprouts if the root crown is killed by fire (Blaisdell & Mueggler, 1956). Low-intensity fires may allow for bitterbrush to sprout; however, community response also depends on soil moisture levels at the time of fire (Murray, 1983). Lower soil moisture allows more charring of the stem below ground level, so sprouting will usually be more successful after a spring fire than after a fire in the summer or fall (Blaisdell & Mueggler, 1956; Murray, 1983; Busse et al., 2000; Kerns et al., 2006). If cheatgrass is present, bitterbrush seedling success is much lower. The factor that most limits establishment of bitterbrush seedlings is competition for water resources with the invasive species cheatgrass (Clements & Young, 2002).
The effect of fire on bunchgrasses relates to culm density, culm-leaf morphology, and the size of the plant. The initial condition of bunchgrasses on the site along with seasonality and intensity of fire all factor into the individual species response. Fore most forbs and grasses, the growing points are located at or below the soil surface. This provides relative protection from disturbances that decrease above ground biomass, such as grazing or fire. Thus, fire mortality is more correlated to duration and intensity of heat, which is related to culm density, culm-leaf morphology, size of plant, and abundance of old growth (Wright, 1971; Young, 1983).
Idaho fescue (Festuca idahoensis), the dominant grass on these communities, response to fire varies with condition and size of the plant, season and severity of fire, and ecological conditions. Mature Idaho fescue plants are commonly severely damaged by fire in all seasons (Wright et al., 1979). Initial mortality may be in excess of 75 percent on severe burns, but usually varies from 20 to 50 percent (Barrington et al., 1989). Rapid burns leave little damage to root crowns, and production of new tillers is triggered by the onset of fall moisture (Johnson et al., 1994). However, Wright et al. (1979) found the dense, fine leaves of Idaho fescue provided enough fuel to burn for hours after a fire had passed, thereby killing or seriously injuring the plant regardless of the intensity of the fire. Idaho fescue is generally more sensitive to fire than the other prominent grasses on these sites such as bluebunch wheatgrass (Pseudoroegneria spicata) (Conrad & Poulton, 1966). However, Robberecht and Defossé (1995) suggested the latter is more sensitive. They observed culm and biomass reduction of bluebunch wheatgrass following moderately severe fire, whereas Idaho fescue required high fire severity for a similar reduction in culm and biomass production. Also, given the same fire severity treatment, post-fire culm production initiated earlier and more rapidly in Idaho fescue.
Fire will remove aboveground biomass from bluebunch wheatgrass, but plant mortality is generally low (Robberecht & Defossé, 1995) because the buds are underground (Conrad & Poulton, 1966) or protected by foliage. Uresk et al. (1976) reported burning increased vegetative and reproductive vigor of bluebunch wheatgrass. Thus, bluebunch wheatgrass is considered to experience slight damage from fire but is more susceptible to fire damage in drought years (Young, 1983).
Conversely, Thurber's needlegrass (Achnatherum thurberianum) is very susceptible to fire-caused mortality. Burning can decrease the vegetative and reproductive vigor of Thurber's needlegrass (Uresk et al., 1976). Fire can cause high mortality in addition to reducing basal area and yield of Thurber's needlegrass (Britton et al., 1990). The fine leaves and densely tufted growth form make this grass susceptible to subsurface charring of the crowns (Wright & Klemmedson, 1965). Although timing of fire highly influences the response and mortality of Thurber's needlegrass, smaller bunch sizes are less likely to be damaged by fire (Wright & Klemmedson, 1965). However, Thurber's needlegrass often survives fire and will continue growth when conditions are favorable (Koniak, 1985). Thus, the intial condition of the bunchgrasses on a site and seasonality and intensity of the fire all factor into the individual species response.
Bluegrasses are a minor component in this group of ecological sites and have been found to increase following fire likely due to their low stature and productivity (Daubenmire, 1975) and may impair establishment of more deeply rooted bunchgrasses.
Livestock/Wildlife Grazing Interpretations:
This group of ecological sites is suitable for grazing. Grazing management considerations include timing, duration and intensity of grazing along with other disturbances that may have changed the resiliency and resistance of the ecological site. In addition, old growth juniper stands provide habitat for a variety of plant and animal species. Bird surveys indicate that the highest abundance and diversity of songbirds occur in shrub steppe communities adjacent to old-growth stands (Reinkensmeyer et al., 2007) but may decline when understory complexity is lost due to canopy closure (Miller et al., 2005).
Domestic sheep, and cattle to a much lesser degree, consume low sagebrush, particularly during the spring, fall, and winter (Sheehy & Winward, 1981). Heavy dormant season grazing by sheep will reduce sagebrush cover and increase grass production (Laycock, 1967). Severe trampling damage to supersaturated soils could occur if sites are used in early spring when there is abundant snowmelt. Trampling damage, particularly from cattle or horses, in low sagebrush habitat types is greatest when highly clayey soils are wet. On drier areas with more gravelly soils, no serious trampling damage occurs, even when the soils are wet (Hironaka et al., 1983). Bunchgrasses, in general, best tolerate light grazing after seed formation. Britton et al. (1990) observed the effects of clipping date on basal area of five bunchgrasses in eastern Oregon and found grazing from August to October (after seed set) has the least impact. Heavy grazing during the growing season will reduce perennial bunchgrasses and increase sagebrush (Laycock, 1967).
Antelope bitterbrush, although a small component of these sites, is a critical browse species for mule deer, antelope, and elk, and is often utilized heavily by domestic livestock (Wood et al., 1995). Grazing tolerance depends on site conditions (Garrison, 1953), and the shrub can be severely hedged during the dormant season for grasses and forbs.
Heavy grazing may lead to replacement of Idaho fescue with non-native species such as cheatgrass (Mueggler, 1975). Bunchgrasses, in general, best tolerate light grazing after seed formation. Britton et al. (1979) observed the effects of harvest date on basal area of five bunchgrasses in eastern Oregon, including Idaho fescue, and found grazing from August to October (after seed set) has the least impact on these bunchgrasses. Therefore, abusive grazing during the growing season will reduce perennial bunchgrasses, except for Sandberg bluegrass (Poa secunda) (Tisdale & Hironaka, 1981). Idaho fescue tolerates light to moderate grazing (Ganskopp & Bedell, 1981) and is moderately resistant to trampling (Cole, 1987). Idaho fescue has been found to decrease under heavy, repeated grazing by livestock (Eckert & Spencer, 1983, 1987; Mueggler, 1975) and wildlife (Gaffney, 1941). However, more recent research by Jaindl et al. (1994) suggests Idaho fescue exhibits overcompensation to single defoliation events (i.e., increased biomass production after herbivory) depending on the phsiological stage of growth at the time of the grazing event.
Bluebunch wheatgrass is moderately grazing tolerant and is very sensitive to defoliation during the active growth period (Blaisdell & Pechanec, 1949; Laycock, 1967; Anderson & Scherzinger, 1975). In studies, herbage and flower stalk production were reduced with clipping at all times during the growing season; clipping was most harmful, however, during the boot stage (Blaisdell & Pechanec, 1949; Britton et al., 1990). Tiller production and growth of bluebunch wheatgrass can be greatly reduced when clipping is coupled with drought (Busso & Richards, 1995). Mueggler (1975) estimated that low-vigor bluebunch wheatgrass may need up to 8 years rest to recover. Although an important forage species, it is not always the preferred species by livestock and wildlife.
Thurber's needlegrass is an important forage source for livestock and wildlife in the arid regions of the West (Ganskopp, 1981). Although the seeds are apparently not injurious, grazing animals avoid them when they begin to mature. Sheep, however, have been oberved grazing the leaves closely, leaving stems untouched (Eckert & Spencer, 1987). Heavy grazing during the growing season has been shown to reduce the basal area of Thurber's needlegrass (Eckert & Spencer, 1987). This suggests that both seasonality and utilization are important factors in management of this plant. A single defoliation, particularly during the boot stage, can reduce herbage production and root mass, thus potentially lowering the competitive ability of this needlegrass (Ganskopp, 1988).
Reduced bunchgrass vigor or density provides an opportunity for Sandberg bluegrass to expand. Sanderbg bluegrass increases under grazing pressue (Tisdale & Hironaka, 1981).
References:
Barrington, M., S. C. Bunting, G. Wright, and I. Moscow. 1989. A fire management plan for Craters of the Moon National Monument. Cooperative Park Studies Unit. Cooperative Agreement CA-9000-8-0005. Moscow, ID: University of Idaho, Rage Resources Department.
Bates, J. D., R. F. Miller, and T. J. Svejcar. 2000. Understory dynamics in cut and uncut western juniper woodlands. Journal of Range Management 53(1):119-126.
Beardall, L. E. and V. E. Sylvester. 1976. Spring burning of removal of sagebrush competition in Nevada. In: Tall Timbers fire ecology conference and proceedings. Tall Timbers Research Station. Vol. 14. Pages 539-547.
Blaisdell, J. P. and J. F. Pechanec. 1949. Effects of herbage removal at various dates on vigor of bluebunch wheatgrass and arrowleaf balsamroot. Ecology 30(3):298-305.
Blaisdell, J. P. and W. F. Mueggler. 1956. Sprouting of bitterbrush (Purshia tridentata) following burning or top removal. Ecology 37(2):365-370.
Blaisdell, J. P., R. B. Murray, and E. D. McArthur. 1982. Managing intermountain rangelands-sagebrush-grass ranges. Gen. Tech. Rep. INT-134. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experimental Station, Ogen, UT. 41 p.
Bradley, A. F., N. V. Noste, and W. C. Fischer. 1992. Fire ecology of forests and woodlands in Utah. Gen. Tech. Rep. INT-287. U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 128 p.
Britton, C. M., G. R. McPherson, and F. A. Sneva. 1990. Effects of burning and clipping on five bunchgrasses in eastern Oregon. Great Basin Naturalist 50(2):115-120.
Britton, C. M., F. A. Sneva, and R. G. Clark. 1979. Effect of harvest date on five bunchgrasses of eastern Oregon. In: 1979 Progress report: Research in Rangeland Management. Special Report 549. Corvallis, OR: Oregon State University, Agricultural Experiment Station: Pages 16-19. In cooperation with: U.S. Department of Agriculture, SEA-AR.
Bunting, S. 1994. Effects of Fire on Juniper woodland ecosystems in the Great Basin. In: S. B. Monsen and S. G. Ketchum, (eds.). Proceedings: Ecology and Management of Annual Rangelands. Gen. Tech. Rep. INT-GTR-313. 1992, May 18-22. U.S. Department of Agriculture, Forest Service, Intermountain Research Station., Boise, ID. Pages 53-55.
Burkhardt, J. W., and E. W. Tisdale. 1976. Causes of juniper invasion in southwestern Idaho. Ecology 57(3):472-484.
Burns, R. M., and B. H. Honkala (Coords.). 1990. Silvics of North America: Volume 1. Conifers; Volume 2. Hardwoods. Agriculture Handbook 654. U.S. Department of Agriculture, Forest Service, Washington, DC. 877 p.
Busse, D., A. Simon, and M. Riegel. 2000. Tree-growth and understory responses to low-severity prescribed burning in thinned Pinus ponderosa forests of central Oregon. Forest Science 46(2):258-268.
Christopherson, J. 2014. Dwarf Mistletoe (Arceuthobium spp.). Nevada Division of Forestry, 2478 Fairview Drive Carson City, Nevada 89701.
Clements, C. D. and J. A. Young. 2002. Restoring Antelope Bitterbrush. Rangelands 24(4):3-6.
Comstock, J. P. and J. R. Ehleringer. 1992. Plant adaptation in the Great Basin and Colorado Plateau. Western North American Naturalist 52(3):195-215.
Daubenmire, R. 1975. Plant succession on abandoned fields, and fire influences in a steppe area in southeastern Washington. Northwest Science 49(1):36-48.
Dobrowolski, J. P., M. M. Caldwell, and J. H. Richards. 1990. Basin hydrology and plant root systems. Pages 243-292 in C. B. Osmond, L. F. Pitelka, and G. M. Hidy (eds.). Plant biology of the basin and range. Springer-Verlag, New York.
Durham, G. 2014. Juniper Pocket Rot (Pyrofomes demidoffii.). Nevada Division of Forestry, 2478 Fairview Drive Carson City, Nevada 89701.
Eddleman, L., P. M. Miller, R. F. Miller, and P. L. Dysart. 1994. Western Juniper Woodlands of the Pacific Northwest: Science Assessment. Department of Rangeland Resources, Oregon State University.
Furniss, M. M. and W. F. Barr. 1975. Insects affecting important native shrubs of the northwestern United States Gen. Tech. Rep. INT-19. U.S. Department of Agriculture, Forest Service, Intermountiain Forest and Range Experiment Station, Ogen, UT. 68 p.
Ganskopp, D. C., and T. E. Bedell. 1981. An assessment of vigor and production of range grasses following drought. Journal of Range Management 34(2):137-141.
Gedney, D. R.; Azuma, D. L.; Bolsinger, C. L., and McKay, N. 1999. Western juniper in eastern Oregon. Gen. Tech. Rep. PNW-GTR-464. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. Portland, OR. 53 p.
Gruell, G. E. 1999. Historical and modern roles of fire in pinyon-juniper. In: S. B. Monsen and R. Stevens, (comps.). Proceedings: Ecology and management of pinyon-juniper communities within the Interior West. 1997, September 15-18. Provo, UT. Proceedings RMRS-P-9. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Provo, UT. Pages 24-28.
Hironaka, M., M. A. Fosberg, and A. H. Winward. 1983. Sagebrush-grass habitat types of southern Idaho. Bulletin Number 35. University of Idaho, Forest, Wildlife and Range Experiment Station, Moscow, ID.
Jaindl, R. G., P. Doescher, R. F. Miller, and L. E. Eddleman. 1994. Persistence of Idaho fescue on degraded rangelands: Adaptation to defoliation or tolerance. Journal of Range Management 47(1):54-59.
Johnson, C. G., R. R. Clausnitzer, P. J. Mehringer, and C. Oilver. 1994. Biotic and abiotic processes of Eastside ecosystems: the effects of management on plant and community ecology, and on stand and landscape vegetation dynamics. Gen. Tech. Rep. PNW-GTR-322. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, OR. 66 p.
Kerns, B. K., W. G. Thies, and C. G. Niwa. 2006. Season and severity of prescribed burn in ponderosa pine forests: implications for understory native and exotic plants. Ecoscience 13(1):44-55.
Kitchen, S. G. and E. D. McArthur. 2007 Big and black sagebrush landscapes. In: S. Hood, M. Miller (eds.). Fire ecoloft and management of the major ecosystems of southern Utah. Gen. Tech. Rep. RMRS-GTR-202. U.S. Department of Agriculture, Forest Serice, Rocky Mountain Research Station, Fort Collins, CO. Pages 73-95.
Koniak, S. 1985. Succession in pinyon-juniper woodlands following wildfire in the Great Basin. The Great Basin Naturalist 45(3):556-566.
McArthur, E. D., H. C. Stutz, S. C. Sanderson. 1983. Taxonomy, distribution, and cytogenetics of Purshia, Cowania, and Fallugia (Rosoideae, Rosaceae). In: A.R. Tiedemann, K. L. Johnson, L. Kendall (eds.), Proceedings- Research and Management of Bitterbrush and Cliffrose in Western North America Gen. Tech. Rep. INT-152, USDA Forest Service Intermountain Forest and Range Experiment Station, Ogden, UT. Pages 4-24.
Miller, R. F., and J. A. Rose. 1995. Historic expansion of Juniperus occidentalis (western juniper) in southeastern Oregon. The Great Basin Naturalist 55(1):37-45.
Miller, R. F., and Tausch, R. J. 2001. The Role of Fire in Juniper and Pinyon Woodlands: A Descriptive Analysis. In: Galley. K. M. and T. P. Wilson (eds.), Fire Conference 2000: The First National Congress on Fire Ecology, Prevention, and Management; Tallahassee, FL: Tall Timbers Research Station, San Diego, CA, USA. Pages 15-30.
Miller, R. F., J. D. Bates, T. J. Svejcar, F. B. Pierson, and L. E. Eddleman. 2005. Biology, ecology, and management of western juniper (Juniperus occidentalis). Oregon State University, Agricultural Experiment Station. Technical Bulletin 152. 77 p.
Miller, R. F., R. J. Tausch, E. D. McArthur, D. D. Johnson, and S. C. Sanderson. 2008. Age structure and expansion of pinon-juniper woodlands: a regional perspective in the Intermountain West. Research Paper RMRS-RP-69. USDA Forest Service, Rocky Mountain Research Station, Fort Collins, CO. 15 p.
Murray, R. B. 1983. Response of antelope bitterbrush to burning and spraying in southeastern Idaho. In: Proceedings: Research and management of bitterbrush and cliffrose in western North America, Salt Lake City. General Technical Report INT-152. Ogden, UT: US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Pages 142-152.
Reinkensmeyer, D. P., Miller, R. F., Anthony, R. G. and V. E. Marr. 2007. Avian community structure along a mountain big sagebrush successional gradient. The Journal of Wildlife Management 71(4):1057-1066.
Rowland, M.M.; Suring, L.H.; Tausch, R. J.; Geer, S. and Wisdom, M. J. 2011. Dynamics of Western Juniper Woodland Expansion into Sagebrush Communities in Central Oregon, Volume 16. Natural Resources and Environmental Issues, Issue 1, Article 13. 12 p.
Soulé, P. T., P. A. Knapp, and H. D. Grissino-Mayer. 2003. Comparative Rates of Western Juniper Afforestation in South- Central Oregon and the Role of Anthropogenic Disturbance. The Professional Geographer 55(1):43-55.
Tausch, R. J. 1999. Historic pinyon and juniper woodland development. In: S. B. Monsen and R. Stevens, (comps.). Proceedings: Ecology and management of pinyon-juniper communities within the Interior West. 1997, September 15-18. Provo, UT. Proceedings RMRS-P-9. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Provo, UT. Pages 12-19.
Tisdale, E. W. and M. Hironaka. 1981. The sagebrush-grass region: A review of the ecological literature. Bulletin 33. University of Idaho, Forest, Wildlife and Range Experiment Station. Moscow, ID. 31p.
Tueller, P. T., and J. E. Clark. 1975. Autecology of pinyon-juniper species of the Great Basin and Colorado Plateau. In: G. F. Gifford and F. E. Busby, (eds.). The pinyon-juniper ecosystem: a symposium. 1975. Utah State University, Logan, UT. Pages 27-40.
Urza, A. K., P. J. Weisberg, J. C. Chambers, J. M. Dhaemers, and D. Board. 2017. Post-fire vegetation response at the woodland-shrubland interface is mediated by the pre-fire community. Ecosphere 8(6):e01851.
Vasek, F. C., and R. F. Thorne. 1977. Transmontane Coniferous Vegetation. Pages 797-832 in M. G. Barbour and J. Major, (eds.), Terrestial vegetation of California. John Wiley & Sons, New York.
West, N. E. 1984. Successional patterns and productivity potentials of pinyon-juniper ecosystems. In: Developing Strategies for Rangeland Management. Westview Press, Boulder, CO. Pages 1301-1332.
Wright, H. A., L. F. Neuenschwander, and C. M. Britton. 1979. The role and use of fire in sagebrush-grass and pinyon- juniper plant communities: A state-of-the-art review. Gen. Tech. Rep. INT-58. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 48 p.
Young, R. P. 1983. Fire as a vegetation management tool in rangelands of the Intermountain region. In: Monsen, S.B. and N. Shaw (eds). Managing Intermountain rangelands—improvement of range and wildlife habitats: Proceedings. 1981, September 15-17; Twin Falls, ID; 1982, June 22-24; Elko, NV. Gen. Tech. Rep. INT-157. Ogden, UT. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Pages 18-31.
Major Land Resource Area
MLRA 023X
Malheur High Plateau
Correlated Map Unit Components
21589615, 21589628, 21589648, 21589588, 21500669, 21500686, 21500630, 21501510, 21501517, 21500728, 21501548, 21501545
Stage
Provisional
Contributors
T Stringham (UNR under contract with BLM)
DMP
Click on box and path labels to scroll to the respective text.