Ecological site group R023XY907OR
Loamy Lake Terraces Basin Big Sagebrush and Indian Ricegrass
Last updated: 06/03/2024
Accessed: 02/27/2026
Ecological site group description
Key Characteristics
None specified
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
Physiography
This group is on lake terraces and plateaus at elevations between 4,000 and 5,500 feet. Slopes are 0 to 15 percent. Slopes less than 5 percent are typical.
Climate
The climate is classified as Cold Semi-Arid in the Koppen Classification System.
The area receives between 8 and 12 inches of annual precipitation as snow in the winter and rain in spring and fall. Summers are generally dry.
The frost-free period is 60 to 115 days. The mean annual air temperature is 45 °F.
Soil features
The soils in this group are shallow to very deep. The textures are loamy or ashy and may have up to 40 percent rock fragments. Soils have a mix of parent materials.
These soils are susceptible to wind erosion and may have small "blow out" areas.
The soil temperature regime is either mesic or frigid. The soils classify as Mollisols and Aridisols. Common soil series in this group are Blayden, Borobey, and Ardep.
Some soils in this group, such as Borobey, have volcanic ash which increases the water holding capacity of those soils.
Vegetation dynamics
Ecological Dynamics and Disturbance Response:
An ecological site is the product of all the environmental factors responsible for its development. Each site has a set of key characteristics that influence its resilience to disturbance and resistance to invasives. According to Caudle et al. (2013), key characteristics include:
1. Climate factors such as precipitation and temperature.
2. Topographic characteristics such as aspect, slope, elevation, and landform.
3. Hydrologic processes such as infiltration and runoff.
4. Soil characteristics such as depth, texture, structure, and organic matter.
5. Plant communities and their functional groups and productivity.
6. Natural disturbance (fire, herbivory, etc.) regime.
Biotic factors that influence resilience include site productivity, species composition and structure, and population regulation and regeneration (Chambers et al., 2013).
The ecological sites in this group are dominated by deep-rooted, cool-season, perennial bunchgrasses and long-lived shrubs (at least 50 years old) with high root to shoot ratios. The dominant shrubs usually root to the full depth of the winter-spring soil moisture recharge, which ranges from 1.0 to over 3.0 meters (Dobrowolski et al., 1990). Root length of mature sagebrush reached a depth of 2 meters in alluvial soils in Utah (Richards & Caldwell, 1987). However, community types with low sagebrush (Artemisia arbuscula) as the dominant shrub had soil depths and thus available rooting depths of 71 to 81 centimeters in a study in northeast Nevada (Jensen, 1990). These shrubs have a flexible generalized root system with development of both deep taproots and laterals near the surface (Comstock & Ehleringer, 1992).
In the Great Basin, the majority of annual precipitation is received during the winter and early spring. This continental semiarid climate regime favors growth and development of deep-rooted shrubs and herbaceous cool-season plants using the C3 photosynthetic pathway (Comstock & Ehleringer, 1992).
Winter precipitation and slow melting of snow results in deeper percolation of moisture into the soil profile. Herbaceous plants, more shallow-rooted than shrubs, grow earlier in the growing season and thrive on spring rains, while the deeper-rooted shrubs lag in phenological development because they draw from deeply infiltrating moisture from snowmelt the previous winter. Periodic drought regularly influences sagebrush ecosystems, and the duration and severity of drought have increased throughout the 20th century in much of the Intermountain West. Major shifts away from historical precipitation patterns have the greatest potential to alter ecosystem function and productivity. Species composition and productivity can be altered by the timing of precipitation and water availability within the soil profile (Bates et al., 2006).
The Great Basin sagebrush communities have high spatial and temporal variability in precipitation both among years and within growing seasons (MacMahon, 1980). Nutrient availability is typically low but increases with elevation and closely follows moisture availability. The invasibility of plant communities is often linked to resource availability. Disturbance changes resource uptake and increases nutrient availability; native species are often damaged and their ability to use resources is depressed for a time, but resource pools may increase from lack of use and/or the decomposition of dead plant material following disturbance (Whisenant, 1999; Miller et al., 2013). The invasion of sagebrush communities by cheatgrass (Bromus tectorum) has been linked to disturbances (fire, abusive grazing) that result in fluctuations in resources (Beckstead & Augspurger, 2004; Chambers et al., 2007; Johnson et al., 2011).
Native insect outbreaks are also important drivers of ecosystem dynamics in sagebrush communities. Climate is generally believed to influence the timing of insect outbreaks, especially outbreaks of a sagebrush defoliator called Aroga moth (Aroga websteri). Aroga moth infestations occurred in the Great Basin in the 1960s, early 1970s, and have been ongoing in Nevada since 2004 (Longland & Young, 1995; Bentz et al., 2008). Thousands of acres of big sagebrush (Artemisia tridentata) have been impacted, with partial to complete die-off observed. The Aroga moth can partially or entirely kill individual plants or entire stands of big sagebrush (Furniss & Barr, 1975). When sagebrush stands are decadent and even-aged, Aroga moth infestations are more likely to be stand-replacing (Longland & Young, 1995).
Indian ricegrass (Achnatherum hymenoides) is the dominant grass on these sites. Indian ricegrass is a deep-rooted, cool-season perennial bunchgrass that is adapted primarily to sandy soils. Grasses generally have shallower root systems than the shrubs on these sites; the root densities of grasses are often as high as or higher than those of shrubs in the upper 0.5 meters of the soil profile, but densities taper off more rapidly than shrubs. The general differences in root depth distributions between grasses and shrubs result in resource partitioning in these shrub/grass systems.
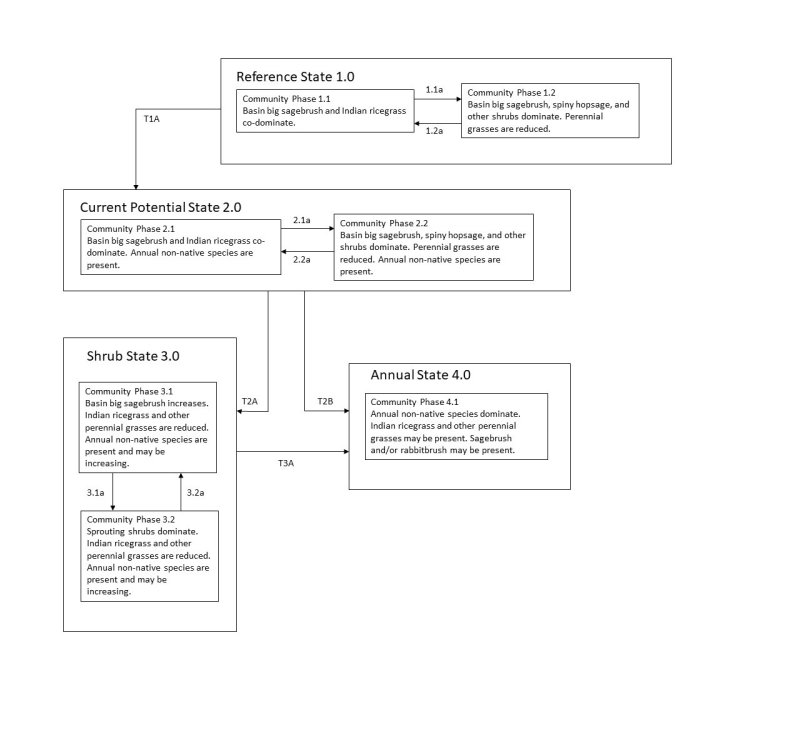
The ecological sites in this group have low to moderate resilience to disturbance and resistance to invasion. Resilience increases with elevation, northerly aspect, precipitation, and nutrient availability. Four possible states have been identified for this group.
Annual Invasive Grasses:
The species most likely to invade these sites are cheatgrass and medusahead (Taeniatherum). Medusahead is more common on clayey soils, so it may never become dominant on sites with loamy soils. This narrative will focus on cheatgrass. Both species are cool-season annual grasses that maintain an advantage over native plants in part because they are prolific seed producers, able to germinate in the autumn or spring, tolerant of grazing, and increase with frequent fire (Klemmedson & Smith, 1964; Miller et al., 1999). Medusahead and cheatgrass originated from Eurasia and both were first reported in North America in the late 1800s (Mack & Pyke, 1983; Furbush, 1953). Pellant and Hall (1994) found 3.3 million acres of public lands dominated by cheatgrass and suggested that another 76 million acres were susceptible to invasion by winter annuals including cheatgrass and medusahead.
Recent modeling and empirical work by Bradford and Lauenroth (2006) suggest that seasonal patterns of precipitation input and temperature are also key factors determining regional variation in the growth, seed production, and spread of invasive annual grasses. Collectively, the body of research suggests that the invasion and dominance of medusahead onto native grasslands and cheatgrass-infested grasslands will continue to increase in severity because conditions that favor native bunchgrasses or cheatgrass over medusahead are rare (Mangla et al., 2011). Medusahead replaces native vegetation and cheatgrass directly by competition and suppression. It replaces native vegetation indirectly by increasing fire frequency.
Methods to control medusahead and cheatgrass include herbicide, fire, grazing, and seeding of primarily non-native wheatgrasses. Mapping potential or current invasion vectors is a management method designed to increase the cost effectiveness of control methods. A study by Davies et al. (2013) found an increase in medusahead cover near roads. Cover was higher near animal trails than random transects, but the difference was less evident. This implies that vehicles and animals aid the spread of the weed; however, vehicles are the major vector of movement. Spraying with herbicide (Imazapic or Imazapic and glyphosate) and seeding with crested wheatgrass (Agropyron cristatum) and Sandberg bluegrass (Poa secunda) have been more successful at combating medusahead and cheatgrass than spraying alone (Sheley et al., 2012). Where native bunchgrasses are missing from the site, revegetation of medusahead- or cheatgrass-invaded rangelands has a higher likelihood of success when using introduced perennial bunchgrasses such as crested wheatgrass (Davies et al., 2015). Butler et al. (2011) tested four herbicides (Imazapic, Imazapic + glyphosate, rimsulfuron, and sulfometuron + Chlorsulfuron), using herbicide-only treatments, for suppression of cheatgrass, medusahead, and ventenata (Ventenata dubia) within residual stands of native bunchgrass. Additionally, they tested the same four herbicides followed by seeding of six bunchgrasses (native and non-native) with varying success. Herbicide-only treatments appeared to remove competition for established bluebunch wheatgrass (Pseudoroegneria spicata) by providing 100 percent control of ventenata and medusahead and greater than 95 percent control of cheatgrass. However, caution in using these results is advised, as only one year of data was reported.
Prescribed fire has also been utilized in combination with the application of pre-emergent herbicide to control medusahead and cheatgrass (J. L. Vollmer & J. G. Vollmer, 2008). Mature medusahead or cheatgrass is very flammable and fire can be used to remove the thatch layer, consume standing vegetation, and even reduce seed levels. Furbush (1953) reported that timing a burn while the seeds were in the milk stage effectively reduced medusahead the following year. He further reported that adjacent unburned areas became a seed source for reinvasion the following year.
When considering the combination of pre-emergent herbicide and prescribed fire for invasive annual grass control, it is important to assess the tolerance of desirable brush species to the herbicide being applied. J. L. Vollmer and J. G. Vollmer (2008) tested the tolerance of mountain mahogany (Cercocarpus montanus), antelope bitterbrush (Purshia tridentata), and multiple sagebrush species to three rates of Imazapic and the same rates with methylated seed oil as a surfactant. They found a cheatgrass control program in an antelope bitterbrush community should not exceed Imazapic at 8 ounces per acre with or without surfactant. Sagebrush, regardless of species or rate of application, was not affected. However, many environmental variables were not reported in this study and managers should install test plots before broad scale herbicide application is initiated.
Fire Ecology:
In many basin big sagebrush (Artemisia tridentata ssp. tridentata) communities, changes in fire frequency co-occurred with fire suppression, livestock grazing, and off-highway vehicle (OHV) use. Few, if any, fire history studies have been conducted on basin big sagebrush. However, Sapsis and Kauffman (1991) suggest that fire return intervals in basin big sagebrush are intermediate between mountain big sagebrush (Artemisia tridentata ssp. vaseyana), 15 to 25 years, and Wyoming big sagebrush (Artemisia tridentata ssp. wyomingensis), 50 to 100 years. Fire severity in big sagebrush communities is “variable” depending on weather, fuels, and topography. However, fire in basin big sagebrush communities is typically stand-replacing (Sapsis & Kauffman, 1991). Basin big sagebrush does not sprout after fire. Because of the time needed to produce seed, it is eliminated by frequent fires (Bunting et al., 1987). Basin big sagebrush reinvades a site primarily from off-site seed or seed from plants that survive in unburned patches. Approximately 90 percent of big sagebrush seed is dispersed within 30 feet (9 meters) of the parent shrub (Goodrich et al., 1985). The maximum seed dispersal is approximately 108 feet (33 meters) from the parent shrub (Shumar & Anderson, 1986). Therefore, regeneration of basin big sagebrush after stand-replacing fires is difficult and dependent upon proximity of residual mature plants and favorable moisture conditions (Johnson & Payne, 1968; Humphrey, 1984).
Spiny hopsage (Grayia spinosa) is a sprouting shrub (Daubenmire, 1970) that is fairly tolerant of fire due its dormancy during the summer months (Rickard & McShane, 1984). After fire, these sprouting shrubs can produce significant new growth if there is enough moisture available (Shaw, 1992). Other environmental conditions such as salinity and soil temperature determine the level of re-establishment that occurs. In order to germinate, seeds need moist conditions (Monsen et al., 2004). Spiny hopsage does not compete well with annual invasives (Monsen et al., 2004).
The effect of fire on bunchgrasses relates to culm density, culm-leaf morphology, and the size of the plant. The initial condition of bunchgrasses on the site and seasonality and intensity of the fire all factor into the individual species response. For most forbs and grasses, the growing points are located at or below the soil surface. This provides relative protection from disturbances that decrease above ground biomass, such as grazing or fire. Thus, fire mortality is more correlated to duration and intensity of heat, which is related to culm density, culm-leaf morphology, size of plant, and abundance of old growth (Wright, 1971; Young, 1983).
Indian ricegrass is fairly fire tolerant (Wright, 1985). This is likely due to its low culm density and below ground plant crowns. Indian ricegrass can reestablish on burned sites through seed dispersed from adjacent unburned areas (Young, 1983; West, 1994). Thus, the presence of surviving, seed-producing plants is necessary for reestablishment of Indian ricegrass. It is important to manage grazing following fire in a way that promotes seed production and establishment of seedlings.
Basin wildrye (Leymus cinereus) is relatively resistant to fire, particularly to fire during the dormant season, as plants sprout from surviving root crowns and rhizomes (Zschaechner, 1985). Miller et al. (2013) reported increased total shoot and reproductive shoot densities in the first year following fire, although by year two there was little difference between burned and control treatments.
The grasses likely to invade the sites of this group are cheatgrass and medusahead. These invasive grasses displace desirable perennial grasses, reduce livestock forage, and accumulate large fuel loads that foster frequent fires (Davies & Svejcar, 2008). Invasion by annual grasses can alter the fire cycle by increasing fire size, fire season length, rate of spread, numbers of individual fires, and likelihood of fires spreading into native or managed ecosystems (D’Antonio & Vitousek, 1992; Brooks et al., 2004). While historical fire return intervals are estimated at 15 to 100 years, areas dominated by cheatgrass are estimated to have a fire return interval of 3 to 5 years (Whisenant, 1990). The mechanisms by which invasive annual grasses alter fire regimes likely interact with climate. For example, cheatgrass cover and biomass vary with climate (Chambers et al., 2007) and are promoted by wet and warm conditions during the fall and spring. Invasive annual species can take advantage of high nitrogen availability following fire because of their higher growth rates and increased seedling establishment relative to native perennial grasses (Monaco et al., 2003).
Livestock/Wildlife Grazing Interpretations:
Personius et al. (1987) found Wyoming big sagebrush and basin big sagebrush to be intermediately palatable to mule deer when compared to mountain big sagebrush (most palatable) and black sagebrush (Artemisia nova) (least palatable).
Spiny hopsage is palatable to livestock, especially sheep, during the spring and early summer (Phillips et al., 1996; Simmons & Rickard, 2003). However, the shrub goes to seed and loses its leaves in July and August, so its usefulness in the fall and winter is limited (Sanderson & Stutz, 1994). Two studies showed little to no utilization by sheep during the winter (Harrison & Thatcher, 1970; Green et al., 1951). Some scientists are concerned about the longevity of the species. One study showed no change in cover or density when excluded from livestock and wildlife grazing for at least 10 years (Rice & Westoby, 1978). Another study seldom observed seedling establishment (Daubenmire, 1970). With poor recruitment rates, some are concerned that repeated fires and overgrazing may eliminate local populations of spiny hopsage (Simmons & Rickard, 2003).
Indian ricegrass is a deep-rooted, cool-season, perennial bunchgrass that is adapted primarily to sandy soils. Indian ricegrass is a preferred forage species for livestock and wildlife (Booth et al., 1980; Cook, 1962). This species is often heavily utilized in winter because it cures well (Booth et al., 2006). It is also readily utilized in early spring because it is a source of green feed before most other perennial grasses have produced new growth (Quinones, 1981). Booth et al. (2006) noted that the plant does well when utilized in winter and spring. However, Cook and Child (1971) found that repeated heavy grazing reduced crown cover, which may reduce seed production, density, and basal area of these plants. Additionally, heavy early spring grazing reduces plant vigor and stand density (Stubbendieck, 1985). In eastern Idaho, productivity of Indian ricegrass was at least 10 times greater in undisturbed plots than in heavily grazed ones (Pearson, 1965). Cook and Child (1971) found significant reduction in plant cover after 7 years of rest from heavy (90 percent vegetation removal) and moderate (60 percent vegetation removal) spring use. The seed crop may be reduced where grazing is heavy (Bich et al., 1995). Tolerance to grazing increases after May, so spring deferment may be necessary for stand enhancement (Cook & Child, 1971; Pearson, 1964). However, utilization of less than 60 percent is recommended.
Basin wildrye is valuable forage for livestock (Ganskopp et al., 2007) and wildlife, but is intolerant of heavy, repeated, or spring grazing (Krall et al., 1971). Basin wildrye is used often as a winter feed for livestock and wildlife since it not only provides roughage above the snow but also cover in the early spring months (Majerus, 1992).
Inappropriate grazing practices can be tied to the success of medusahead, but eliminating grazing will not eradicate medusahead if it is already present (Wagner et al., 2001). Sheley and Svejcar (2009) reported that even moderate defoliation of bluebunch wheatgrass resulted in increased medusahead density. They suggested that disturbances such as plant defoliation limit soil resource capture, which creates an opportunity for exploitation by medusahead. Avoidance of medusahead by grazing animals allows medusahead populations to expand. This creates seed reserves that can infest adjoining areas and cause changes to the fire regime. Medusahead replaces native vegetation and cheatgrass directly by competition and suppression; it replaces native vegetation indirectly by an increase in fire frequency.
Medusahead litter has a slow decomposition rate because of its high silica content, allowing it to accumulate over time and suppress competing vegetation (Bovey et al., 1961; Davies & Johnson, 2008).
References:
Beckstead, J., and Augspurger, C. K. 2004. An experimental test of resistance to cheatgrass invasion: limiting resources at different life stages. Biological Invasions 6(4):417-432.
Bentz, B., D. Alston, and T. Evans. 2008. Great Basin Insect Outbreaks. In: J. Chambers, N. Devoe, A. Evenden (eds.). Collaborative Management and Research in the Great Basin -- Examining the issues and developing a framework for action Gen. Tech. Rep. RMRS-GTR-204. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, CO. Pages 45-48.
Bich, B. S., J. L. Butler, and C. A. Schmidt. 1995. Effects of differential livestock use on key plant species and rodent populations within selected Oryzopsis hymenoides/Hilaria jamesii communities of Glen Canyon National Recreation Area. The Southwestern Naturalist 40(3):281-287.
Booth, D. T., C. G. Howard, and C. E. Mowry. 1980. 'Nezpar' Indian ricegrass: description, justification for release, and recommendations for use. Rangelands 2(2):53-54.
Booth, E. G., J. F. Mount, and J. H. Viers. 2006. Hydrologic variability of the Cosumnes River floodplain. San Francisco Estuary and Watershed Science 4(2):1-19. Article 2.
Bovey, W. R., D. Le Tourneau, and C. L. Erickson. 1961. The chemical composition of medusahead and downy brome. Weeds 9(2):307-311.
Bradford, J. B., and W. K. Lauenroth. 2006. Controls over invasion of Bromus tectorum: The importance of climate, soil, disturbance and seed availability. Journal of Vegetation Science 17(6):693-704.
Brooks, M. L., C. M. D'Antonio, D. M. Richardson, J. B. Grace, J. E. Keeley, J. M. Ditomaso, R. J. Hobbs, M. Pellant, and D. Pyke. 2004. Effects of Invasive Alien Plants on Fire Regimes. BioScience 54(7):677-688.
Butler, M., R. Simmons, and F. Brummer. 2011. Restoring Central Oregon Rangeland from Ventenata and Medusahead to a Sustainable Bunchgrass Environment – Warm Springs and Ashwood. Central Oregon Agriculture Research and Extension Center. COARC 2010. Pages 77-82.
Comstock, J. P. and J. R. Ehleringer. 1992. Plant adaptation in the Great Basin and Colorado plateau. Western North American Naturalist 52(3):195-215.
D'Antonio, C. M., and P. M. Vitousek. 1992. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review of Ecology and Systematics 23:63-87.
Daubenmire, R. 1970. Steppe vegetation of Washington. Technical Bulletin 62. Washington Agriculture Experiment Station. 131 p.
Davies, K. W., and D. D. Johnson. 2008. Managing medusahead in the intermountain west is at a critical threshold. Rangelands 30(4):13-15.
Davies, K. W., and T. J. Svejcar. 2008. Comparison of medusahead-invaded and noninvaded Wyoming big sagebrush steppe in southeastern Oregon. Rangeland Ecology and Management 61(6):623-629.
Davies, K. W., A. M. Nafus, and M. D. Madsen. 2013. Medusahead invasion along unimproved roads, animal trails, and random transects. Western North American Naturalist 73(1):54-59.
Davies, K. W., C. S. Boyd, D. D. Johnson, A. M. Nafus, and M. D. Madsen. 2015. Success of seeding native compared with introduced perennial vegetation for revegetating medusahead-invaded sagebrush rangeland. Rangeland Ecology & Management 68(3):224-230.
Dobrowolski, J. P., M. M. Caldwell, and J. H. Richards. 1990. Basin hydrology and plant root systems. Pages 243-292 in C. B. Osmond, L. F. Pitelka, and G. M. Hidy (eds.). Plant biology of the basin and range. Springer-Verlag, New York.
Furbush, P. 1953. Control of Medusa-Head on California Ranges. Journal of Forestry 51(2):118-121.
Furniss, M. M. and W. F. Barr. 1975. Insects affecting important native shrubs of the northwestern United States Gen. Tech. Rep. INT-19. Intermountain Forest and Range Experiment Station, U.S. Department of Agriculture, Forest Service. Ogden, UT. 68 p.
Goodrich, S., E. D. McArthur, and A. H. Winward. 1985. A new combination and a new variety in Artemisia tridentata. The Great Basin Naturalist 45(1):99-104.
Harrison, B. J., and A. P. Thatcher. 1970. Winter sheep grazing and forage preference in southwestern Wyoming. Journal of Range Management 23(2):109-111.
Johnson, B. G.; Johnson, D. W.; Chambers, J. C.; Blank, B.R. 2011. Fire effects on the mobilization and uptake of nitrogen by cheatgrass (Bromus tectorum L.). Plant and Soil 341(1-2):437-445.
Klemmedson, J. O., and J. G. Smith. 1964. Cheatgrass (Bromus Tectorum L.). The Botanical Review 30(2):226-262.
Longland, W. S., and J. A. Young. 1995. Landscape Diversity in the Western Great Basin. In: N. E. West, (ed.). Biodiversity on Rangelands, proceedings of the symposium. 1993, February 16. Albuquerque, New Mexico. College of Natural Resources, Utah State University, Logan, UT. Pages 80-91.
Mack, R. N., and D. Pyke. 1983. The Demography of Bromus Tectorum: Variation in Time and Space. Journal of Ecology 71(1):69-93.
MacMahon, J. A. 1980. Ecosystems over time: succession and other types of change. In: Waring, R., ed. Proceedings—Forests: fresh perspectives from ecosystem analyses. Biological Colloquium. Corvallis, OR: Oregon State University. Pages 27-58.
Majerus, M. E. 1992. High-stature grasses for winter grazing. Journal of Soil and Water Conservation 47(3):224-225.
Mangla, S., R. Sheley, and J. J. James. 2011. Field growth comparisons of invasive alien annual and native perennial grasses in monocultures. Journal of Arid Environments 75(2):206-210.
Miller, H. C., Clausnitzer, D., and Borman, M. M. 1999. Medusahead. In: R. L. Sheley and J. K. Petroff (eds.). Biology and Management of Noxious Rangeland Weeds. Corvallis, OR: Oregon State University Press. Pages 272-281.
Miller, R. F., J. C. Chambers, D. A. Pyke, F. B. Pierson, and C. J. Williams. 2013. A Review of Fire Effects on Vegetation and Soils in the Great Basin Region: Response and Ecological Site Characteristics. Gen. Tech. Rep. RMRS-GTR-308. Fort Collins CO: U.S. Department of Agriculture, United States Forest Service, Rocky Mountain Research Station, Fort Collins, CO. 126 p.
Monaco, T. A., C. T. Mackown, D. A. Johnson, T. A. Jones, J. M. Norton, J. B. Norton, and M. G. Redinbaugh. 2003. Nitrogen effects on seed germination and seedling growth. Journal of Range Management 56(6):646-653.
Monsen, S. B., R. Stevens, and N. L. Shaw. 2004. Grasses. Pages 295-424 in Restoring western ranges and wildlands, vol. 2. Gen. Tech. Rep. RMRS-GTR-136-vol-2. USDA: Forest Service, Rocky Mountain Research Station, Fort Collins, CO.
Pellant, M., and C. Hall. 1994. Distribution of two exotic grasses in intermountain rangelands: status in 1992. USDA Forest Service Gen. Tech. Rep. INT-GTR-313S. Pages 109-112.
Phillips, R. L., N. K. McDougald, and J. Sullins. 1996. Plant preference of sheep grazing the Mojave desert. Rangelands 18(4):141-144.
Quinones, F. A. 1981. Indian ricegrass evaluation and breeding. Bulletin 681. New Mexico State University, Agricultural Experiment Station, Las Cruces, NM. Page 19.
Rickard, W., and M. McShane. 1984. Demise of spiny hopsage shrubs following summer wildfire: An authentic record. Northwest Science 58(4):282-285.
Sanderson, S. C., and H. C. Stutz. 1994. Woody chenopods useful for rangeland reclamation in western North America. In: S. B. Monsen and S. G. Kitchen, (eds.). Proceedings: Ecology and Management of Annual Rangelands. Gen. Tech. Rep. INT-GTR-313. 1992, May 18-22. Boise, ID. U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Ogden, UT. Pages 374-378.
Shaw, N. L. 1992. Germination and Seedling Establishment of Spiny Hopsage (Grayia spinosa [Hook.] Moq.). Thesis. Ph. D. Oregon State University.
Sheley, R. L., Svejcar T. J. 2009. Response of bluebunch wheatgrass and medusahead to defoliation. Rangeland Ecology & Management 62(3):278-283.
Sheley, R. L., E. A. Vasquez, A. Chamberlain, and B. S. Smith. 2012. Landscape-scale rehabilitation of medusahead (Taeniatherum caput-medusae)-dominated sagebrush steppe. Invasive Plant Science and Management 5(4):436-442.
Simmons, S. A., and W. H. Rickard. 2003. Fire effects on spiny hopsage in south central Washington. Western North American Naturalist 63(4):524-528.
Stubbendieck, J. L. 1985. Nebraska Range and Pasture Grasses: (including Grass-like Plants). University of Nebraska, Department of Agriculture, Cooperative Extension Service, Lincoln, NE. 75 p.
Vollmer, J. L., and J. G. Vollmer. 2008. Controlling cheatgrass in winter range to restore habitat and endemic fire United States Department of Agriculture, Forest Service. RMRS-P-52. Pages 57-60.
Wagner, J. A., R. E. Delmas, J. A. Young. 2001. 30 years of medusahead: return to fly blown-flat. Rangelands 23(3):6-9.
Whisenant, S., 1999. Repairing Damaged Wildlands: a process-orientated, landscape-scale approach (Vol. 1). Cambridge, UK: Cambridge University Press. 312 p.
Wright, H. A. 1985. Effects of fire on grasses and forbs in sagebrush-grass communities. In: K. D. Sanders and J. Durham, (eds.). Rangeland Fire Effects; A Symposium. 1984, November 27-29. USDI-BLM, Boise, ID. Pages 12-21.
Young, R. P. 1983. Fire as a vegetation management tool in rangelands of the Intermountain region. In: Monsen, S.B. and N. Shaw (eds). Managing Intermountain rangelands—improvement of range and wildlife habitats: Proceedings. 1981, September 15-17; Twin Falls, ID; 1982, June 22-24; Elko, NV. Gen. Tech. Rep. INT-157. Ogden, UT. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Pages 18-31.
Zschaechner, G. A. 1985. Studying rangeland fire effects: a case study in Nevada. In: K. Sanders and J. Durham, (eds.). Rangeland Fire Effects: A Symposium. 1984, November 27-29. USDI-BLM Idaho State Office, Boise, ID. Pages 66-84.
Major Land Resource Area
MLRA 023X
Malheur High Plateau
Subclasses
Correlated Map Unit Components
21659413, 21659129, 22170817, 22170836, 22170933, 22170932, 22168238, 22168422, 22175606, 22176830, 22176526, 22175680, 22175614, 22176525, 22176228, 22176229, 22175952, 22177512, 22177511
Stage
Provisional
Contributors
T Stringham (UNR under contract with BLM)
DMP
Click on box and path labels to scroll to the respective text.