Ecological site group R023XY917NV
Mountain Slope 16-20 PZ Mahogany
Last updated: 06/03/2024
Accessed: 02/21/2026
Ecological site group description
Key Characteristics
- Site does not pond or flood
- Landform other than dunes
- Surface soils are not clayey
- Sites are tree dominated
- Elevations < 7000'
- Soils loamy or ashy
- Frost free days < 100
- Soils > 12" deep.
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
Physiography
This group is on mountain slopes at elevations between 5,500 and 9,400 feet. Slopes are 15 to 50 percent.
Climate
The climate is classified as Cold Semi-Arid in the Koppen Classification System.
The area receives 16 to 24 inches annual precipitation as snow in the winter and rain in the spring and fall. Summers are generally dry.
The frost-free period is 40 to 70 days.
Soil features
The soils in this group are loamy-skeletal or ashy-skeletal. Soil depth may be very shallow to deep. Very shallow soils in this group have a paralithic contact. Soil temperature regimes are principally cryic.
Typically, the surface has considerable amounts of rock fragments. Taxonomically, these soils are Mollisols.
The common soil series in this group are Badgercamp, Cowbell, and Zorromount.
Vegetation dynamics
Ecological Dynamics and Disturbance Response:
An ecological site is the product of all the environmental factors responsible for its development. Each site has a set of key characteristics that influence its resilience to disturbance and resistance to invasives. According to Caudle et al. (2013), key characteristics include:
1. Climate factors such as precipitation and temperature.
2. Topographic characteristics such as aspect, slope, elevation, and landform.
3. Hydrologic processes such as infiltration and runoff.
4. Soil characteristics such as depth, texture, structure, and organic matter.
5. Plant communities and their associated functional groups and productivity.
6. Natural disturbance (fire, herbivory, etc.) regime.
Biotic factors that influence resilience include site productivity, species composition and structure, and population regulation and regeneration (Chambers et al., 2013).
The Great Basin vegetative communities have high spatial and temporal variability in precipitation both among years and within growing seasons (MacMahon, 1980). Nutrient availability is typically low but increases with elevation and closely follows moisture availability. Water stored in the soil profile during winter is the moisture resource that supports most plant growth. Disturbance changes resource uptake and increases nutrient availability, often to the benefit of non-native species; native species are often damaged and their ability to use resources is depressed for a time, but resource pools may increase from lack of use and/or the decomposition of dead plant material following disturbance (Whisenant, 1999; Miller et al., 2013). The invasion of cheatgrass (Bromus tectorum) has been linked to disturbances (fire, abusive grazing) that result in fluctuations in resources (Chambers et al., 2007). Dobrowolski et al. (1990) cite multiple authors on the extent of the soil profile exploited by the competitive, exotic annual cheatgrass. Specifically, the root depth of cheatgrass depends on the size the plant. In competitive environments, cheatgrass roots were found to penetrate only 15 centimeters; roots of isolated plants and pure stands were measured to at least 1 meter, with some plants rooting as deep as 1.5 to 1.7 meters.
Long-lived curl-leaf mountain mahogany (Cercocarpus ledifolius), deep-rooted, cool-season perennial bunchgrasses, and long-lived shrubs (at least 50 years old) with high root to shoot ratios dominate the ecological sites in this group. The dominant shrubs usually root to the full depth of the winter-spring soil moisture recharge, which ranges from 1.0 to over 3.0 meters (Comstock & Ehleringer, 1992). Root length of mature sagebrush plants was measured to a depth of 2 meters in alluvial soils in Utah (Richards & Caldwell, 1987). These shrubs have a flexible generalized root system with development of both deep taproots and laterals near the surface (Comstock & Ehleringer, 1992). The perennial bunchgrasses generally have somewhat shallower root systems than the shrubs on these sites; root densities of perennial bunchgrasses are often as high as or higher than those of shrubs in the upper 0.5 meters. The general differences in root depth distributions between grasses and shrubs result in resource partitioning in this system.
Curl-leaf mountain mahogany is a multi-branched, evergreen shrub or tree extending from 3 to over 20 feet in height. The roots of curl-leaf mountain mahogany are spreading and limited by the depth to bedrock. Youngberg and Hu (1972) reported in an Oregon study that curl-leaf mountain mahogany produces nitrogen-fixing root nodules. They also reported that nodulated plants had the highest amounts of nitrogen in the leaves. It is the most widely distributed species of Cercocarpus and is the only species of the genus that extends as far north and west as Washington. Curl-leaf mountain mahogany stands most often grow on warm, dry, rocky ridges or outcrops where fire is infrequent (USDA, 1988). Dealy (1974) and Scheldt (1969) found that curl-leaf mountain mahogany trees were larger and older on fire-resistant rocky sites. Trees in these positions become seed sources if fire destroys the non-rocky portion of a site.
Curl-leaf mountain mahogany plants are long-lived and can surpass 1,300 years of age (Schultz, 1987; Schultz et al., 1990). As curl-leaf mountain mahogany stands increase in average age, the average canopy volume and height of the individuals present also increase. As average canopy height and volume increase, stand density declines (Schultz et al., 1991). Stands with a closed, or nearly closed canopy often have few or zero young curl-leaf mountain mahogany individuals (i.e., recruitment) in the understory (Schultz et al., 1990, 1991), despite high seed density beneath trees (Russell & Schupp, 1998, Ibáñez & Schupp, 2002). Intraspecific competition reduces the growth rates of all age classes below the potential growth rates for the species. Competition may also increase mortality in the younger plants.
Once germination occurs, the seedlings exhibit rapid growth in relation to top growth, providing some resistance to drought and competition with invasive species (Dealy, 1974). Dealy (1974) reported that curl-leaf mountain mahogany seedlings have a mean taproot length of 0.97 meters after 120 days. The mean height was slightly less than 2.5 centimeters. Multiple sources (Ibáñez et al., 1999; Schultz et al., 1996) found that curl-leaf mountain mahogany seedlings germinate abundantly under the canopy of adult plants but rarely successfully establish there due to shading and higher litter amounts. In addition, Schultz et al. (1996) found that seedlings had significantly higher long-term success in areas dominated by sagebrush canopy than in areas under curl-leaf mountain mahogany canopy or in interspaces. Some hypothesize that the light shading and hydraulic lift provided by sagebrush may create a microsite that facilitates curl-leaf mountain mahogany recruitment (Gruell et al., 1985; Ibáñez et al., 1999).
Curl-leaf mountain mahogany stands are susceptible to drought, frost, and invasion by non-native species, especially cheatgrass. Cheatgrass affects curl-leaf mountain mahogany seedling growth by competing for water resources and nutrients (Ross, 1999).
Mountain big sagebrush (Artemisia tridentata ssp. vaseyana) is generally long-lived; therefore, it is not necessary for new individuals to recruit every year for perpetuation of the stand. Infrequent, large recruitment events and simultaneous low, continuous recruitment are the foundation of population maintenance (Noy-Meir, 1973). Survival of the seedlings depends on adequate moisture conditions.
The perennial bunchgrasses that are co-dominant with the shrubs on these sites include bluebunch wheatgrass (Pseudoroegneria spicata) and Idaho fescue (Festuca idahoensis). These species generally have somewhat shallower root systems than the shrubs on these sites; root densities of bluebunch wheatgrass and Idaho fescue are often as high as or higher than those of shrubs in the upper 0.5 meters but densities taper off more rapidly than shrubs. The differences in root depth distributions between grasses and shrubs result in resource partitioning in these shrub/grass systems.
Letterman’s needlegrass (Achnatherum lettermanii), the dominant grass on the non-modal ecological site, is an erect, densely tufted perennial bunchgrass that forms large clumps. It grows on dry soils in a variety of vegetation communities, including high elevation meadows, subalpine grasslands, open areas underneath aspen, and sagebrush communities. It grows best on loamy soils that are greater than 20 centimeters deep (Dittberner & Olson, 1983).
Cusick’s bluegrass (Poa cusickii) and/or muttongrass (Poa fendleriana) are on the sites of this group. There is evidence that these two common names have been used interchangeably (Monsen et al., 2004) or are sometimes misidentified, but they occupy similar ecological niches (Cronquist et al., 1994). Cusick’s bluegrass is a strongly tufted perennial grass but may be somewhat rhizomatous in loose soils (Cronquist et al., 1994). It begins growth very early in the season and may produce two crops of inflorescences in a growing season (Cronquist et al., 1994).
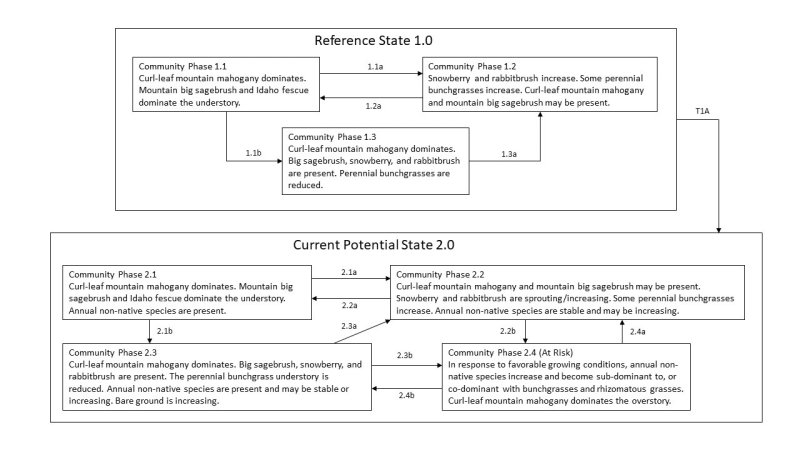
The ecological sites in this group have moderate to high resilience to disturbance and resistance to invasion. Resilience increases with elevation, northerly aspect, precipitation, and nutrient availability. Long-term disturbance response may be influenced by small differences in landscape topography. North slopes are more resilient than south slopes because lower soil surface temperatures operate to keep moisture content higher on northern exposures. Two possible alternative stable states have been identified for this group.
Fire Ecology:
The fire return interval in curl-leaf mountain mahogany-dominated sites is not well documented. However, a study by Arno and Wilson (1986) suggests sites of curl-leaf mountain mahogany with ponderosa pine (Pinus ponderosa) had fire return intervals of 13 to 22 years before 1900. Fire frequency most likely depends on surrounding vegetation. Most often, curl-leaf mountain mahogany stands are on warm, dry, rocky ridges or outcrops where fire is infrequent (USDA, 1988). Dealy (1974) and Scheldt (1969) found that curl-leaf mountain mahogany trees are larger and older on fire-resistant rocky sites and are seed sources if fire destroys the non-rocky portion of a site. Curl-leaf mountain Mahogany will persist longest in rocky areas where it is protected from fire. Because of their thicker bark, mature trees can often survive low-severity fires (Gruell et al., 1985). Curl-leaf mountain mahogany sprouts weakly after fire. It is usually moderately to severely damaged by severe fires and the recovery time of these sites is variable; some measurements show that stands lack recruitment for up to 30 years post-fire (Gruell et al., 1985).
Mountain big sagebrush is killed by fire (Neuenschwander, 1980; Blaisdell et al., 1982), and does not resprout (Blaisdell, 1953). Post-fire regeneration starts from seed and varies depending on site characteristics, seed source, and fire characteristics. Mountain big sagebrush seedlings can grow rapidly and may reach reproductive maturity within 3 to 5 years (Bunting et al., 1987). Mountain big sagebrush may return to pre-burn density and cover within 15 to 20 years following fire, but establishment after severe fires may proceed more slowly (Bunting et al., 1987).
Depending on fire severity, snowberry (Symphoricarpos sp.) and other sprouting shrubs may increase after fire. Snowberry is top-killed by fire, but resprouts after fire from rhizomes (Leege & Hickey, 1971; Noste & Bushey, 1987). Snowberry has been noted to regenerate well and exceed pre-burn biomass in the third season after a fire (Merrill et al., 1982). Yellow rabbitbrush (Chrysothamnus viscidiflorus) is also on these sites. It has a large taproot root system and is known to be shorter lived and less competitive than sagebrush. Seedling density, flower production, and shoot growth decline as competition from other species increases (McKell & Chilcote, 1957; Miller et al. 2013). Yellow rabbitbrush is top-killed by fire, but sprouts vigorously after fire (Kuntz, 1982; Akinsoji 1988). If balsamroot (Balsamorhiza sp.) or mule-ears (Wyethia sp.) are common before fire, these plants will increase after fire or with heavy grazing (Wright, 1985).
Idaho fescue response to fire varies with condition and size of the plant, season and severity of fire, and ecological conditions. Idaho fescue can generally survive low-severity fires but can be severely damaged by fire in all seasons (Wright et al., 1979; Wright, 1985). Rapid burns leave little damage to root crowns, and production of new tillers corresponds with the onset of fall moisture (Johnson et al., 1994). However, another study found the dense, fine leaves of Idaho fescue provided enough fuel to burn for hours after a fire had passed, thereby killing or seriously injuring the plant regardless of the intensity of the fire (Wright et al., 1979). Rapid tillering can occur after fire when root crowns are not killed and soil moisture is favorable (Johnson et al., 1994; Robberecht & Defossé, 1995).
Fire will remove aboveground biomass from bluebunch wheatgrass but plant mortality is generally low (Robberecht & Defossé, 1995) because the buds are underground (Conrad & Poulton, 1966) or protected by foliage. However, season and severity of the fire will influence plant response. Plant response will vary depending on post-fire soil moisture availability. Letterman’s needlegrass recovers well after fire (Monsen et al., 2004). Burning reduces the basal area and flower stalk production of Cusick’s bluegrass (Uresk et al., 1976). In the same study, burning enhanced the growth of bluebunch wheatgrass.
Livestock/Wildlife Grazing Interpretations:
Curl-leaf mountain mahogany is an important cover and browse species for big game such as elk (Cervus canadensis), mule deer (Odocoileus heminous), pronghorn antelope (Antilocarpra americana), and bighorn sheep (Ovis canadensis) (Lanner, 1984; Furniss et al., 1988; Sabo et al., 2005). Sampson and Jespersen (1963) state that curl-leaf mountain mahogany is excellent browse for mule deer, and domestic livestock will browse this plant to varying degrees in all seasons except summer. It is not uncommon for these trees to develop a “hedged” appearance after years of regular browsing by wildlife. According to Olsen (1992), curl-leaf mountain mahogany is consumed widely by mule deer throughout the year. In fact, mule deer fecal pellets contained curl-leaf mountain mahogany year-round, with the highest frequency of leaves found in winter (Gucker, 2006). Mule deer will use curl-leaf mountain mahogany for cover as well (Steele et al., 1981).
Despite low palatability, mountain big sagebrush is eaten in small amounts by sheep, cattle, goats, and horses. Chemical analysis indicates that the leaves of big sagebrush equal alfalfa meal in protein, have a higher carbohydrate content, and yield twelvefold more fat (USDA, 1988).
Antelope bitterbrush (Purshia tridentata) is a small component of these sites, but is a critical browse species for mule deer, antelope, and elk and is often utilized heavily by domestic livestock (Wood et al., 1995). Grazing tolerance depends on site conditions (Garrison, 1953) and the shrub can be severely hedged during the dormant season for grasses and forbs.
Idaho fescue is valuable forage for livestock and wildlife. It is an excellent forage grass and can withstand heavy trampling (USDA, 1988). However, Idaho fescue decreases under heavy grazing by livestock (Eckert & Spencer, 1987) and wildlife (Gaffney, 1941).
Bluebunch wheatgrass is moderately grazing-tolerant and is very sensitive to defoliation during the active growth period (Blaisdell & Pechanec, 1949; Laycock, 1967; Anderson & Scherzinger, 1975). In studies, herbage and flower stalk production were reduced with clipping at all times during the growing season; clipping was most harmful, however, during the boot stage (Blaisdell & Pechanec, 1949; Britton et al., 1990) Tiller production and growth of bluebunch wheatgrass were greatly reduced when clipping was coupled with drought (Busso & Richards, 1995). Mueggler (1975) estimated that low-vigor bluebunch wheatgrass may need up to 8 years rest to recover. Although an important forage species, it is not always the preferred species by livestock and wildlife.
Letterman’s needlegrass provides valuable forage for both livestock and wildlife (Taylor, 2000). It begins growth early in the year and is available to be utilized when other grasses are not yet palatable. It is especially important fall forage for big game (Monsen et al., 2004). Letterman’s needlegrass has been shown to increase under sheep grazing and decreases under light cattle and horse grazing (Bowns & Bagley, 1986). It also declines when grazing is excluded for a long time (Turner, 1969).
Cusick’s bluegrass was the most palatable and preferred grass compared to Thurber’s needlegrass (Achnatherum thurberianum) and bluebunch wheatgrass in a grazing study, but was also the most negatively affected by grazing (Rickard et al., 1975). Uresk and Rickard (1976) found Cusick’s bluegrass to be a highly preferred grass, especially in the spring, even when it is a minor component of the plant community.
References:
Akinsoji, A. 1988. Postfire vegetation dynamics in a sagebrush steppe in southeastern Idaho, USA. Vegetation 78(3):151-155.
Blaisdell, J. P. 1953. Ecological Effects of Planned Burning of Sagebrush-Grass Range on the Upper Snake River Plains. Technical Bulletin No. 1075, USDA, Washington, D.C.
Blaisdell, J. P. and J. F. Pechanec. 1949. Effects of Herbage Removal at Various Dates on Vigor of Bluebunch Wheatgrass and Arrowleaf Balsamroot. Ecology 30(3):298-305.
Blaisdell, J. P., R. B. Murray, and E. D. McArthur. 1982. Managing intermountain rangelands-sagebrush- grass ranges. Gen. Tech. Rep. INT-134. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station, Ogden, UT. 41 p.
Bowns, J. E. and Bagley, C. F. 1986. Vegetation responses to long term sheep grazing on mountain ranges. Journal of Range Management 39(5):431-434.
Britton, C. M., G. R. McPherson, and F. A. Sneva. 1990. Effects of burning and clipping on five bunchgrasses in eastern Oregon. Great Basin Naturalist 50(2):115-120.
Comstock, J. P. and J. R. Ehleringer. 1992. Plant adaptation in the Great Basin and Colorado Plateau. Western North American Naturalist 52(3):195-215.
Cronquist, A., A. H. Holmgren, N. H. Holmgren, J. L. Reveal, and P. K. Holmgren. 1994. Intermountain Flora Vascular Plants of the Intermountain West, U.S.A. The New York Botanical Garden, Bronx, New York. 584 p.
Dealy, J. E. 1974. Ecology of curl-leaf mountain-mahogany (Cercocarpus ledifolius Nutt.) in eastern Oregon and adjacent areas. Unpublished dissertation. Oregon State University, Corvallis, OR. 176 p.
Dittberner, P. L.; Olsen, M. R. 1983. The Plant Information Network (PIN) database: Colorado, Montana, North Dakota, Utah, and Wyoming. FWS/OBS-83/36. Washington, DC: U.S. Department of Interior, Fish and Wildlife Service, Division of Biological Services, Research and Development, Western Energy and Land Use Team. 786 p.
Dobrowolski, J. P., M. M. Caldwell, and J. H. Richards. 1990. Basin hydrology and plant root systems. Pages 243-292. In C. B. Osmond, L. F. Pitelka, and G. M. Hidy (Eds.), Plant biology of the basin and range. Springer-Verlag, New York.
Furniss, M. M., D. C. Ferguson, K. W. Voget, J. W. Burkhardt, A. R. Tiedemann, and J. L. Oldemeyer. 1988. Taxonomy, Life History, and Ecology of a Mountain-mahogany Defoliator, Stamnodes animata (Pearsall), in Nevada. Fish and Wildlife Research; 3. U.S. Department of the Interior, Fish and Wildlife Service, Washington, DC. 26 p.
Gruell, G., S. C. Bunting, and L. Neuenschwander. 1985. Influence of fire on curlleaf mountain-mahogany in the Intermountain West. In: J. E. Lotan and J. K. Brown, (comps.). Fire's Effects on Wildlife Habitat - Symposium Proceedings. Gen. Tech. Rep. INT-186. 1984, March 21. Missoula, Montana. USDA Forest Service, Intermountain Research Station, Pages 58-72.
Gucker, C. L. 2006. Cercocarpus ledifolius. Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory.
Ibáñez, I., E. W. Schupp, and J. L. Boettinger. 1999. Successional History of a Curlleaf Mountain Mahogany Stand: a Hypothesis. In E. D. McArthur, W. K. Ostler, and C. L. Wambolt (Eds.), Proceedings: shrubland ecotones. Proc. RMRS-P-11. 1998, August 12-14. Ephraim, UT. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Ogden, UT. Pages 102-107.
Johnson, C. G., Jr., R. R. Clausnitzer, P. J. Mehringer, and C. Oliver. 1994. Biotic and abiotic processes of Eastside ecosystems: the effects of management on plant and community ecology and on stand and landscape vegetation dynamics. Gen. Tech. Rep. PNW-GTR-322. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 66 p.
Kuntz, D. E. 1982. Plant response following spring burning in an Artemisia tridentata subsp. vaseyana/Festuca idahoensis habitat type. Dissertation, University of Idaho, Moscow, ID.
Lanner, R. M. 1984. Trees of the Great Basin: A Natural History. University of Nevada Press, Reno, NV. 215 p.
MacMahon, J. A. 1980. Ecosystems over time: succession and other types of change. In R. Waring (Ed.), Proceedings--Forests: fresh perspectives from ecosystem analyses. Biological Colloquium. Corvallis, OR: Oregon State University. Pages 27-58.
Miller, R. F., J. C. Chambers, D. A. Pyke, F. B. Pierson, and C. J. Williams. 2013. A Review of Fire Effects on Vegetation and Soils in the Great Basin Region: Response and Ecological Site Characteristics. Gen. Tech. Rep. RMRS-GTR-308. Fort Collins CO: U.S. Department of Agriculture, United States Forest Service, Rocky Mountain Research Station, Fort Collins, CO. 126 p.
Monsen, S.B., R. Stevens, N.L. Shaw, (comps.). 2004. Restoring western ranges and wildlands. Gen. Tech. Rep. RMRS-GTR-136. Volume 2. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. Pages 295-698 plus index.
Noste, N. V. and C. L. Bushey. 1987. Fire response of shrubs of dry forest habitat types in Montana and Idaho. General Technical Report INT-239. USDA Forest Service, Intermountain Research Station, Ogden, UT. 22 p.
Olsen, R. 1992. Mule deer habitat requirements and management in Wyoming. B-965. University of Wyoming, Cooperative Extension Service, Laramie, WY. 21 p.
Rickard, W. H., D. W. Uresk, and J. F. Cline. 1975. Impact of Cattle Grazing on Three Perennial Grasses in South-Central Washington. Journal of Range Management 28(2):108-112.
Ross, C. 1999. Population dynamics and changes in Curl-leaf Mountain Mahogany in two adjacent Sierran and Great Basin mountain ranges. Ph.D. Dissertation. University of Nevada, Reno.
Russell, S., and E. Schupp. 1998. Effects of Microhabitat Patchiness on Patterns of Seed Dispersal and Seed Predation of Cercocarpus ledifolius (Rosaceae). Oikos 81(3):434-443.
Sampson, A.W., B.S. Jespersen. 1963. California range brushlands and browse plants. Berkeley, CA: University of California, Division of Agricultural Sciences, California Agricultural Experiment Station, Extension Service. 162 p.
Scheldt, R. S. 1969. Ecology and utilization of curl-leaf mountain mahogany in Idaho. Unpublished Thesis. University of Idaho, Moscow, ID.
Schultz, B.W. 1987. Ecology of curl-leaf mountain mahogany (Cercocarpus ledifolius) in western and central Nevada: population structure and dynamics. Unpublished M.S. Thesis. University of Nevada Reno.
Schultz, B. W., R. J. Tausch, and P. T. Tueller. 1991. Size, age, and density relationships to curl-leaf mahogany (Cercocarpus ledifolus) populations in western and central Nevada: competitive implications. Great Basin Naturalist 51(2):183-191.
Schultz, B. W., R. J. Tausch, and P. T. Tueller. 1996. Spatial relationships among young Cercocarpus ledifolius (curl-leaf mountain mahogany). Great Basin Naturalist 56(3):261-266.
Steele, R., R. D. Pfister, R. A. Ryker, and J. A. Kittams. 1981. Forest habitat types of central Idaho. Gen. Tech. Rep. INT-114. U. S. Department of Agriculture, Forest Service, Intermountain Research Station, Ogden, UT. 138 p.
Taylor, J. L. 2000. Achnatherum lettermanii. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/
Turner, G. T. 1969. Responses of mountain grassland vegetation to gopher control, reduced grazing, and herbicide. Journal of Range Management 22(6):377-383.
Uresk, D.W., and Rickard, W.H. 1976. Diets of steers on a shrub-steppe rangeland in south-central Washington. Journal of Range Management 29(6):464-466.
[USDA] United States Department of Agriculture. 1988. Range Plant Handbook (Reproduction of the 1937 edition). Dover Publications, Inc.: New York. 848 p.
Wood, M. K., Bruce A. Buchanan, & William Skeet. 1995. Shrub preference and utilization by big game on New Mexico reclaimed mine land. Journal of Range Management 48(5):431-437.
Wright, H. A. 1985. Effects of fire on grasses and forbs in sagebrush-grass communities. In K. D. Sanders and J. Durham (Eds.), Rangeland Fire Effects; A Symposium. 1984, November 27-29. USDI-BLM, Boise, ID. Pages 12-21.
Wright, H. A., C. M. Britton, and L. F. Neuenschwander. 1979. The role and use of fire in sagebrush-grass and pinyon-juniper plant communities: A state-of-the-art review. Gen. Tech. Rep. INT-58. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 48 p.
Youngberg, C. T., and L. Hu. 1972. Root nodules on mountain mahogany. Forest Science 18(3):211-212.
Major Land Resource Area
MLRA 023X
Malheur High Plateau
Subclasses
Correlated Map Unit Components
21500675, 21501468, 21501492, 21501534, 21501532, 21501526, 21501457, 21500994, 21500898, 21500902, 21589650, 21590030, 21589694, 21589887, 21590073, 21589483, 21589886, 21590069, 21590806, 21590309, 21590707, 21590393, 21604613, 21604594, 21604596, 22171079, 22170895
Stage
Provisional
Contributors
T Stringham (UNR under contract with BLM)
DMP
Click on box and path labels to scroll to the respective text.