Ecological site group F023XY923NV
Cold North Slope Quaking Aspen
Last updated: 06/03/2024
Accessed: 02/11/2026
Ecological site group description
Key Characteristics
- Site does not pond or flood
- Landform other than dunes
- Surface soils are not clayey
- Sites are tree dominated
- Elevation > 7000'
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
Physiography
This group is on concave mountain slopes, principally on northern aspects, at elevations above 6,000 feet. Slopes are 4 to 75 percent.
The sites receive supplemental moisture as snowmelt runoff from the upper slopes.
Climate
The climate is classified as Cold Semi-Arid in the Koppen Classification System. The area receives between 14 and 20 inches of annual precipitation, principally as snow. Summers are generally dry. The frost-free period is less than 70 days per year, and the mean annual air temperature is 40 to 45 °F.
Soil features
The soils in this group are deep, loamy soils formed from alluvium, colluvium, or loess. The soil temperature regime is cryic. The soils classify as Mollisols. Common soil series in this ecological site group are Hackwood, Hashwood, and Tosp.
Vegetation dynamics
An ecological site is the product of all the environmental factors responsible for its development. Each site has a set of key characteristics that influence its resilience to disturbance and resistance to invasives. According to Caudle et al. (2013), key characteristics include:
1. Climate factors such as precipitation and temperature.
2. Topographic characteristics such as aspect, slope, elevation, and landform.
3. Hydrologic processes such as infiltration and runoff.
4. Soil characteristics such as depth, texture, structure, and organic matter.
5. Plant communities and their functional groups and productivity.
6. Natural disturbance (fire, herbivory, etc.) regime.
Biotic factors that influence resilience include site productivity, species composition and structure, and population regulation and regeneration (Chambers et al., 2013).
Aspen is the most widely distributed tree in North America. In the West it is the only upland hardwood tree (Monsen et al., 2004). Aspen is typically found in nearly pure stands. Cryer and Murray (1992) found that stable aspen stands survive only on soils with a mollic horizon. Lateral roots may extend over 30 meters, with vertical sinker roots reaching nearly 3 meters deep. Entire stands are often a single clone from root sprouts or suckers. In such stands, individual “trees” are known as ramets. Aspen can establish from seed, but reproduction is primarily by root sprouts that develop within 10 meters of the parent stem. Growth from primordia (root tissue) is suppressed until the tree is top-killed by fire or another disturbance; just girdling the trees does not promote root sprouts (Perala, 1990).
Individual trees are short lived (less than 150 years) and rely on regular disturbances to regenerate (Bartos & Mueggler, 1981; Shepperd & Smith, 1993). Aspen is shade intolerant, which promotes even-aged ramets. Stands of mixed-age trees only form under stable conditions where the overstory gradually dies off with disease or age, and is replaced by aspen suckers (Perala, 1990).
Common disturbances in aspen stands include fire, insect and disease outbreaks, windstorms, and avalanches. Aspen stands have also shown some sensitivity to drought (Hogg et al., 2008). Quaking aspen (Populus tremuloides) is one of the most widely distributed forest plants in North America (Potter, 1998). Mature aspen stands (80 to 100 years) can reach heights up to 100 feet depending on the site. Most stands contain a variety of medium-high shrubs and tall herbs in the understory (DeByle & Winokur, 1985). Increased fire suppression, excessive browse pressure, and conifer encroachment threaten the structure of aspen stands in the West.
Conifer Dynamics:
Shading by conifer trees limits aspen regeneration (Bartos & Campbell, 1998; Stringham et al., 2015). If the aspen stands are near or intermixed with conifers like white fir (Abies concolor), spruce (Picea sp.), pinyon (Pinus sp.), or juniper (Juniperus sp.), the clone is at risk of being overtopped and killed from competition and shading over time (Wall et al., 2001). There is widespread encroachment of western juniper (Juniperus occidentalis) in northwestern Great Basin aspen stands; as juniper cover increases in these areas, aspen tree density, recruitment, and herbaceous understory production declines (Wall et al., 2001; Miller et al., 2000). The increase in conifers can be attributed to both fire suppression and grazing pressure by both livestock and wildlife (Potter, 2005; Strand et al., 2009b; Bartos & Campbell, 1998). Using a habitat model, Strand et al. (2009a) computed aspen occurrence probability across the landscape of the Owyhee Plateau. They visited 41 sites where they modeled aspen occurrence: 37 percent had dead aspen stems with no aspen regeneration; 51 percent had scattered aspen ramets and aspen regeneration in forest gaps; and 12 percent of the sites showed no evidence that aspen had ever occurred on or near the site. Strand et al.’s aspen successional model theorized that non-producing aspen stands can be permanently converted to a conifer stand and the aspen clone can be lost. They estimated that over 60 percent of aspen woodlands have been, or are in the process of being, converted to conifer woodlands. It is unknown how long an aspen stand can survive in a non-reproductive state under conifer canopy closure (Strand et al., 2009a).
Overstory clearing, whether in small gaps or in large openings, provides the needed light for aspen suckers to sprout (Shepperd et al., 2006). A limited aspen root system resulting from previous conifer dominance and/or persistent shading from surrounding uncut trees may require additional disturbance to initiate suckering. Additional management actions such as root ripping may be needed to stimulate root suckering (Shepperd et al., 2006). Prescribed fire is an effective tool for removing western juniper and releasing aspen stands; fall burning is most effective in removing juniper (Bates & Davies, 2018a), while spring burning has more desirable effects on the understory (Bates & Davies, 2018b). Other studies have explored this technique for releasing aspen and have seen success (Bartos & Mueggler, 1981; Brown & DeByle, 1989; DeByle, 1985; Walker, 1993). Limiting browse impacts is crucial to allow aspen regeneration after disturbance (see Livestock Interpretations section below).
There are many environmental factors that can contribute to stand decline or die-off. The major underlying cause can be attributed to tree and/or stand stress. Drought, low soil oxygen, and cold soil temperatures all limit soil water uptake and can contribute to xylem cavitation. Cavitation causes much of the aspen die-off, but the resulting stress can also leave the stand prone to secondary factors such as wood-boring insects and fungal pathogens (Frey et al., 2004). Drought is also attributed to the decline and death of aspen trees, and similarly contributes to secondary factors such as insects (Frey et al., 2004).
Aspen stands possess three characteristics that provide suitable sites for invasive plants:
1. Deep, rich soils.
2. Proximity to moist meadows and riparian areas with open water.
3. Dependency on disturbance and open light.
The ecological sites of this group are moderately resilient to disturbance and resistant to invasion. Human disturbance associated with recreation and animal (domestic and wildlife) disturbance may lead to the spread of invasive species such as Kentucky bluegrass (Poa pratensis), common dandelion (Taraxacum officinale), and thistles (Cirsium spp.).
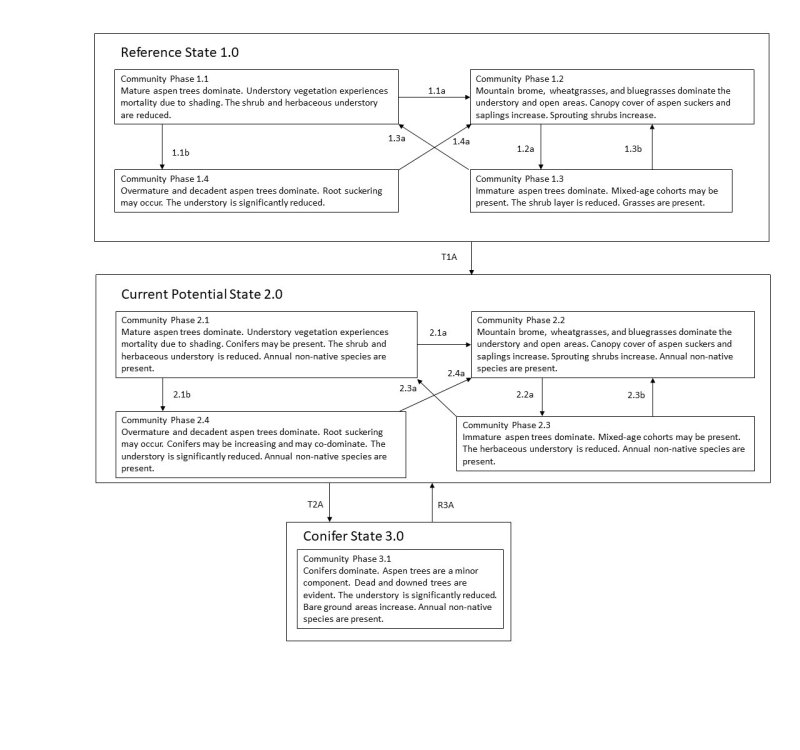
The ecological sites contained within this group are moderately resilient and resistant due to productive soils, additional soil moisture, and the ability of aspen to sprout following fire or other stand or tree removal processes. Three stable states have been identified for this group: a reference state, a current potential state, and a tree state.
Fire Ecology:
Wildfire is a natural disturbance that influences the structure and composition of the vegetation of the reference state. Periodic wildfires prevent over-mature aspen stands and maintain a naturally stratified mosaic of even- aged aspen communities in various stages of successional development (Strand et al., 2009b). Wall et al. (2001) found a pattern of even-aged aspen stands that indicated there were stand-replacing fires roughly every 16 to 17 years on average. Aspen can regrow even when subjected to fires only 3 years apart (Perala, 1990). Although aspen stands rely on fire for successful regeneration, aspen stands don’t readily carry fire alone (Fechner & Barrows, 1976; DeByle & Winokur, 1985; DeByle et al., 1987; Monsen et al., 2004). At least 80 percent top-kill may be necessary to promote suckering (Brown, 1985). Bates and Davies (2018a) used cut and dried juniper to carry prescribed fire through experimental aspen stands. Aspen is extremely fire sensitive (Baker, 1925). Due to aspen’s thin bark, most individual ramets are killed by fire, and those left with scarring are usually killed within the next growing season by rot and disease (Bradley et al., 1992; Davidson et al., 1959; Meinecke, 1929). However, fires that kill the aspen overstory usually stimulate abundant suckering and enhance the long-term health of the clone (DeByle & Winokur, 1985; Bartos & Mueggler, 1981; Turner et al., 2003).
It is hypothesized that many of the fires that maintained these communities were set by the Native American population to manage plant communities for human benefit (Kay, 1997). Specific fire intervals depend upon surrounding vegetation communities. Reduced fire intervals in the last 100 to 150 years threaten the survival of existing aspen stands; fire suppression is a factor in reducing aspen recruitment (Hessl, 2002). Historic heavy grazing has been attributed to the reduction of fine fuels within stands; without the fuels to burn, fires seldom occur within aspen forests (DeByle & Winokur, 1985). While wild or prescribed fire can be a tool to promote aspen regeneration and clone health, it is important to manage browse impacts or else the beneficial effects of fire may be negated (Smith et al., 2016).
Mountain big sagebrush (Artemisia tridentata ssp. vaseyana), a minor component on these sites, is killed by fire (Neuenschwander, 1980; Blaisdell et al., 1982), and does not resprout (Blaisdell, 1953). Post-fire regeneration from seed and varies depending on site characteristics, seed source, and fire characteristics. Mountain big sagebrush seedlings can grow rapidly and may reach reproductive maturity within 3 to 5 years (Bunting et al.,1987). Mountain big sagebrush may return to pre-burn density and cover within 15 to 20 years following fire, but establishment after severe fires may proceed more slowly and can take up to 50 years (Bunting et al., 1987; Ziegenhagen, 2003; Miller & Heyerdahl, 2008; Ziegenhagen & Miller, 2009).
Mountain snowberry (Symphoricarpos oreophilus) is top-killed by fire, but resprouts after fire from rhizomes (Leege & Hickey, 1971; Noste & Bushey, 1987). Snowberry has been noted to regenerate well and exceed pre-burn biomass in the third season after fire (Merrill et al., 1982). Currant (Ribes sp.), a minor component of these sites, sprouts weakly from the root crown, but usually regenerates from soil-stored seeds after fire. It is susceptible to fire mortality and rarely survives fire (Crane & Fischer, 1986). Utah serviceberry (Amelanchier utahensis) sprouts after fire (Conrad, 1987) and grows more rapidly than some other serviceberry species (Plummer et al.,1968). If balsamroot (Balsamorhiza sp.) or mule-ears (Wyethia sp.) are common on the site before fire, these plants will increase after fire or heavy grazing (Wright, 1985).
The effect of fire on bunchgrasses relates to culm density, culm-leaf morphology, and the size of the plant. The initial condition of bunchgrasses on the site along with seasonality and intensity of the fire all factor into the individual species response. For most forbs and grasses, the growing points are located at or below the soil surface. This provides relative protection from disturbances that decrease above ground biomass, such as grazing or fire. Thus, fire mortality is more correlated to duration and intensity of heat, which is related to culm density, culm-leaf morphology, size of plant, and abundance of old growth (Wright, 1971; Young, 1983).
Mountain brome (Bromus marginatus), the dominant grass found on these sites, is a robust, coarse-stemmed, short-lived perennial bunchgrass that can grow 1 to 5 feet tall (USDA, 1988; Tilley et al., 2004a). Mountain brome significantly decreases after burning (Nimir & Payne, 1978), but is commonly seeded after wildfires in high elevation areas, due to its ability to establish quickly from seed and reduce erosion (Tilley et al., 2004a). Slender wheatgrass (Elymus trachycaulus), a sub-dominant grass on these sites, may increase after fire. In a study by Nimir and Payne (1978), slender wheatgrass increased significantly on burned sites compared to non-burned sites, although the species did not appear in measurable quantities until mid-July after a spring (May) burn in the same year.
Livestock Grazing/Wildlife Browse Interpretations:
These sites are valuable for livestock grazing and wild ungulate browse. Grazing considerations include timing, intensity, and duration of grazing. Domestic livestock, wild ungulates, rodents, and rabbits utilize aspen stands and can have a measurable impact on them (Kay & Bartos, 2000). Cattle have a less injurious effect on aspen sprouts than sheep, who more readily browse twigs (Sampson, 1919). However, cattle and sheep still use aspen significantly less than deer and elk (Beck & Peek, 2005). Browsing during the sapling stage reduces aspen growth, vigor, and numbers (DeByle & Winokur, 1985). Heavy browsing on aspen suckers may result in lower clone vigor to the point that suckering no longer takes place (Lindroth & St. Clair, 2013). Browsing pressure may allow aspen to regenerate but prevents the development of trees, causing aspen to grow as a dense shrub instead of a tree (Bradley et al. 1992), or resulting in an aspen stand consisting only of old age classes with many dead stems (Hessl, 2002). A study of aspen across Utah, Idaho, and Wyoming showed that only 2 percent of trees were less than 50 years old, indicating that the effect of increasing elk numbers along with effects of cattle and deer use have prevented recruitment over time (Mueggler, 1989).
Snowberry is an important forage plant for sheep, deer, elk, and bighorn sheep (Guillon, 1964). Snowberry is poor to fair browse for cattle but may be heavily used by domestic livestock on overgrazed ranges (Morris et al., 1962). Utah serviceberry is considered a staple browse for deer and livestock, while the fruits are preferred by birds and small mammals (Conrad, 1987). Utah serviceberry also constituted two percent of the stomach contents of a big horn ram taken out of Clark County in 1952 (Guillon, 1964).
Mountain brome increases with grazing (Leege et al., 1981). A study by Mueggler (1967) found that with clipping, mountain brome increased in herbage production when clipped in June. When clipped in July, mountain brome increased due to reduced competition from forb species. The study also found that after 3 successive years of clipping, mountain brome started to show adverse effects. Mountain brome is ranked as highly valuable for elk winter forage (Kufeld, 1973).
Slender wheatgrass is a perennial bunchgrass that tends to be short lived but spreads well by natural reseeding (Monsen et al., 2004). It is widely used in restoration seedings (Monsen et al., 2004). Slender wheatgrass tends to persist for a longer time than other perennial grasses when subjected to heavy grazing (Monsen et al., 1996, 2004). Slender wheatgrass is palatable and nutritious for livestock. It is also grazed by wild ungulates and used for cover by small birds and mammals (Tilley et al., 2004b; Hallsten et al., 1987).
The forb community (Lupinus spp. and Balsamorhiza sagitata) found in aspen stands is important forage for sheep, cattle, deer, and elk (Beck & Peek, 2005).
Wildlife Interpretations:
Aspen stands are valued for their ability to support greater plant, insect, and bird biodiversity compared to surrounding forests and shrublands (Chong et al., 2001; Griffis-Kyle & Beier, 2003). These sites provide valuable habitat for several species of wildlife. Quaking aspen is important forage for large mammals. Elk (Alces alces) browse the bark, branches, and sprouts of quaking aspen year-round throughout the West (DeByle, 1979; Howard, 1996). Mule deer (Odocoileus hemionus) use quaking aspen year-round, especially if winters are mild. They browse leaves, buds, twigs, bark, and sprouts. New growth after burns or clearcuts are readily consumed by mule deer (Innes, 2013). Moose (Alces americanus) are only occasionally seen in Nevada but will feed on the bark of quaking aspen in winter, the saplings in spring, and leaves and branches the rest of the year (Shepperd et al., 2006). Black bear (Ursus americanus) will eat stems and leaves of quaking aspen, but forbs and other plants found in the quaking aspen understory are preferred (Beetle, 1974; Wildlife Action Plan Team, 2012). A study by Krebill (1972) found the majority of aspen decline within their study area was due to a combination of pathogenic fungi and insects that invade aspen trees damaged by big game.
Several lagomorphs use quaking aspen habitat. Although aspen groves are at elevations where desert cottontail (Sylvilagus audubonii) are not normally found, desert cottontail may use aspen habitat where aspen groves occur at lower elevations with sagebrush and shrubland (DeByle & Winokur, 1985). Snowshoe hares (Lepus americanus) feed on quaking aspen in summer and spring and will continue to use quaking aspen habitat year-round but are more common in the associated coniferous forests (DeByle & Winokur, 1985). The threatened species American Pika (Ochotona princeps) utilizes quaking aspen stands in higher elevation habitats, and has been documented feeding on quaking aspen buds, twigs, and bark (Wildlife Action Plan Team, 2012; Howard, 1996).
Rodents utilize aspen habitat for food and cover. Mountain pocket gophers (Thomomys monticola), a fossorial rodent, favor quaking aspen stands (Cassola, 2016). Aspen soils rarely freeze which makes them ideal for burrowing. Forbs and aspen sprouts provide forage in the spring and summer (DeByle & Winokur, 1985). Deer mice (Peromyscus maniculatus) and least chipmunks (Tamias minimus) occupy quaking aspen habitat (DeByle 1979). The deer mouse was trapped more than any other rodent, consistently throughout several years, in quaking aspen stands according to Andersen et al. (1980). The least chipmunk has been trapped at near equal density as the deer mouse in aspen habitat (DeByle & Winokur, 1985; Andersen et al., 1980). The Inyo shrew (Sorex tenellus), Merriam’s shrew (Sorex merriami), montane shrew (Sorex monticolus), and western jumping mouse (Zapus princeps) use the shrub and herbaceous cover within quaking aspen habitat for foraging and cover (Wildlife Action Plan Team, 2012). The flying squirrel (Glaucomys sabrinus), although rarely seen because of its nocturnal habit, is estimated to be one of the most common mammal species found in aspen woodlands (DeByle & Winokur, 1985). Larger rodents, such as the North American porcupine (Erethizon dorsatus) will eat quaking aspen in winter and spring months. In winter, porcupine eat the smooth outer bark of the upper trunk and branches; in spring, they eat the buds and twigs (Howard, 1996; DeByle & Winokur, 1985).
Beaver (Castor canadensis) use a large amount of aspen for building material to construct their dams. In fact, as many as 200 quaking aspen stems are required to support 1 beaver for a 1-year period. Beaver prefer the inner bark of aspen to that of other trees as food (Lanner, 1984). They will consume the leaves, bark, twigs, and quaking aspen branches of any diameter (Innes, 2013). Previous research estimated that an individual beaver consumes 2 to 4 pounds (1 to 2 kilograms) of quaking aspen bark daily (DeByle & Winokur, 1985).
Quaking aspen provides feed and cover for a variety of bird species in Nevada. The northern goshawk (Accipiter gentilis) and flammulated owl (Psiloscops flammeolus) use mature overstory for nesting (Wildlife Action Plan Team, 2012). Bird species including orange-crowned and yellow-rumped warblers (Vermivora celata and Dendroica coronata, respectively), broad-tailed hummingbirds (Selasphorus platycercus), robins (Turdus migratorius), house wrens (Troglodytes aedon), pewees (Contopus sordidulus), juncos (Junco hyemalis), and thrushes (Catharus ustulatus) nest and forage aspen stands. Furthermore, dead trees are used by downy woodpeckers (Picoides pubescens), flickers (Colaptes auratus), and Lewis’ woodpeckers (Melanerpes lewis) (Lanner, 1984; Wildlife Action Plan Team, 2012). Birds such as the mountain bluebird (Sialia currucoides), tree swallow (Tachycineta bicolor), pine siskin (Spinus pinus), and black-headed grosbeak (Pheucticus melanocephalus) can be found at the edges of aspen communities (Flack, 1976). Even duck species, including wood duck (Aix sponsa), common and barrow’s goldeneye (Bucephala clangula and Bucephala islandica, respectively), bufflehead (Bucephala albeola), and hooded and common merganser (Lophodytes cucullatus and Mergus merganser, respectively) utilize aspen habitat (DeByle & Winokur, 1985). Dusky grouse (Dendragapus obscurus), sooty grouse (Dendragapus fuliginosus), mountain quail (Oreortyx pictus), and rufous hummingbird (Selasphorus rufus) utilize the shrub and herbaceous cover provided by quaking aspen forests (Wildlife Action Plan Team, 2012).
Several bat species live within subalpine habitats, adding to the communities’ diversity. The fringed myotis (Myotis thysanodes), long-eared myotis (myotis evotis), hoary bat (Lasiurus cinereus), silver-haired bat (Lasionycteris noctivagans), little brown myotis (Myotis lucifugus), and western small-footed myotis (Myotis ciliolabrum) all are documented as occurring in quaking aspen forests and meadows above 9,000 feet (Keinath, 2003; Arroyo-Calbrales & Álvarez-Castañeda, 2008; Warner & Czaplewski, 1984; Sullivan, 2009; Wildlife Action Plan Team, 2012).
Habitat distribution of reptiles and amphibians is not as widely studied as other animals, and few reptiles and amphibians are found at elevations where quaking aspen trees grow. However, the Columbia spotted frog (Rana luteiventris) and northern rubber boa (Charina bottae) favor downed quaking aspen trees as well as stored ground moisture maintained by dead, decomposing logs (Wildlife Action Plan Team, 2012).
Threats and Management:
Problems contributing to the decline of aspen communities in Nevada include fire suppression, conifer encroachment, improper livestock grazing, and browsing by big game species (Wildlife Action Plan Team, 2012). Several fungi species cause large cankers on aspen trunks and roots and spots on leaves. The fungus Marssonina leaf blight causes particular damage to the trees by leaving brown leaves on quaking aspen mid-summer throughout large portions of their habitat (Lanner, 1984).
References:
Andersen, D. C., J. A. MacMahon, and M. L. Wolfe. 1980. Herbivorous Mammals along a Montane Sere: Community Structure and Energetics. Journal of Mammalogy 61(3):500-519.
Arroyo-Cabrales, J. and Álvarez-Castañeda, S. T. 2008. Myotis evotis. The IUCN Red List of Threatened Species. Version 2014. 3. Downloaded on 23 January 2015.
Baker, F. S. 1925. Aspen in the Central Rocky Mountain Region. Department Bulletin No. 1291. United States Department of Agriculture, Washington, D.C.
Bartos, D. L., and Mueggler, W. F. 1981. Early succession in aspen communities following fire in western Wyoming. Journal of Range Management 34(4):315-318.
Bartos, D. L., and R. B. Campbell, Jr. 1998. Decline of Quaking Aspen in the Interior West-Examples from Utah. Rangelands 20(1):17-24.
Bates, J. D., and Davies, K. W. 2018a. Quaking aspen woodland after conifer control: Tree and shrub dynamics. Forest Ecology and Management 409:233-240.
Bates, J. D., and Davies, K. W. 2018b. Quaking aspen woodland after conifer control: Herbaceous dynamics. Forest Ecology and Management 409:307-316.
Beck, J. L., and Peek, J. M. 2005. Diet composition, forage selection, and potential for forage competition among elk, deer, and livestock on aspen-sagebrush summer range. Rangeland Ecology & Management 58(2):135-147.
Beetle, A. A. 1974. The zootic disclimax concept. Journal of Range Management 27(1):30-32.
Blaisdell, J. P. 1953. Ecological Effects of Planned Burning of Sagebrush-Grass Range on the Upper Snake River Plains. Technical Bulletin No. 1075. USDA, Washington, D.C.
Blaisdell, J. P., R. B. Murray, and E. D. McArthur. 1982. Managing intermountain rangelands-sagebrush- grass ranges. Gen. Tech. Rep. INT-134. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station, Ogden, UT. 41p.
Bradley, A. F., N. V. Noste, and W. C. Fischer. 1992. Fire ecology of forests and woodlands in Utah. Gen. Tech. Rep. INT-287. U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 128p.
Brown, J. K., and DeByle, N. V. 1989. Effects of prescribed fire on biomass and plant succession in western aspen. Research Paper INT-412. U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Ogden, UT. 16p.
Brown, R. F. 1985. The growth and survival of young mulga (Acacia aneura F. Muell) trees under different levels of grazing. Australian Rangeland Journal 7(2):143-148.
Cassola, F. 2016. Thomomys monticola. The IUCN Red List of Threatened Species 2016. https://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T42596A22216069.en. Accessed on 18 April 2023.
Chong, G. W., S. E. Simonson, T. J. Stohlgren, and M. A. Kalkhan. 2001. Biodiversity: Aspen stands have the lead, but will nonnative species take over? In: W. D. Shepperd, D. Binkley, D. L. Bartos, T. J. Stohlgren, and L. G. Eskew, (comps.). Sustaining aspen in western landscapes: Symposium proceedings. Proceedings RMRS-P-18. 2000, June 13-15. Grand Junction, CO. USDA, Forest Service, Rocky Mountain Research Station, Fort Collins, CO. Pages 261-272.
Conrad, E. 1987. Common shrubs of chaparral and associated ecosystems of southern California. Gen. Tech. Rep. PSW-99. United States Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 87p.
Crane, M. F., and W. C. Fischer. 1986. Fire Ecology of the Forest Habitat Types of Central Idaho. Gen. Tech. Rep. INT-218. USDA-Forest Service, Intermountain Research Station, Ogden, UT. 86p.
Cryer, D. H., and J. E. Murray. 1992. Aspen regeneration and soils. Rangelands 14(4):223-226.
Davidson, R. W., T. E. Hinds, and F. G. Hawksworth. 1959. Decay of Aspen in Colorado. Station Paper No. 45. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, CO. 14p.
DeByle, N. V. 1979. Potential effects of stable versus fluctuating elk populations in the aspen ecosystem. Aspen Bibliography Paper 4689.
DeByle, N. V. 1985. Managing wildlife habitat with fire in the aspen ecosystem. In: J. E. Lotan and J. K. Brown, (comps.). Symposium: Fire's effects on wildlife habitat. Gen. Tech. Rep. INT-GTR-186. 1984, March 21. Missoula, MT. U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Ogden, UT. Pages 73-82.
DeByle, N. V., and R. P. Winokur, editors. 1985. Aspen: ecology and management in the western United States. USDA Forest Service Gen. Tech. Rep. RM-119. Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colo. 283p.
Fechner, G. H., and Barrows, J. S. 1976. Aspen stands as wildfire fuel breaks. Aspen bibliography. Paper 5029.
Flack, J. A. D. 1976. Bird Populations of Aspen Forests in Western North America. Ornithological Monographs 19:1-97.
Griffis-Kyle, K. L., and P. Beier. 2003. Small isolated aspen stands enrich bird communities in southwestern ponderosa pine forests. Biological Conservation 110(3):375-385.
Guillon, G.W. 1964. Wildlife use of Nevada plants. Contributions toward a flora of Nevada No. 49. Beltsville, MD: U.S. Department of Agriculture, Agriculture Research Service, Plant Industry Station, Crops Research Division. 161p.
Hallsten, G. P., Q. D. Skinner, A. A. Beetle. 1987. Grasses of Wyoming. 3rd edition. University of Wyoming, Agricultural Experiment Station, Laramie, WY. 432p.
Hessl, A., 2002. Aspen, Elk, and Fire: The Effects of Human Institutions on Ecosystem Processes: The interactions among aspen, elk, and fire in the intermountain West highlight important mismatches between ecological processes and human institutions and provide important insights for the management of national parks and other protected areas. AIBS Bulletin 52(11):1011-1022.
Hogg, E. H, J. P. Brandt, and M. Michaelin. 2008. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests. Canadian Journal of Forest Research. 38(6):1373-1376.
Howard, J. L. 1996. Populus tremuloides. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory.
Innes, R. J. 2013. Odocoileus hemionus. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory.
Kay, C. E. 1997. Is Aspen doomed? Journal of Forestry 95(8):4-11.
Kay, C. E., and Bartos, D. L. 2000. Ungulate herbivory on Utah aspen: assessment of long-term exclosures. Journal of Range Management 53(2):145-153.
Keinath, D. A. 2003. Species assessment for fringed Myotis (Myotis thysanodes) in Wyoming. United State Department of the Interior, Bureau of Land Management. Cheyenne, WY. 71p.
Krebill, R. G. 1972. Mortality of aspen on the Gros Ventre elk winter range. Aspen Bibliography. Paper 5398. http://digitalcommons.usu.edu/aspen_bib/5398
Lanner, R. M. 1984. Trees of the Great Basin: a natural history. University of Nevada Press, Reno, NV.
Lindroth, R. L. and St. Clair, S. B. 2013. Adaptations of quaking aspen (Populus tremuloides Michx.) for defense against herbivores. Forest Ecology and Management 299:14-21.
Meinecke, E. P. 1929. Quaking aspen: A study in applied forest pathology. Technical Bulletin 155. U.S. Department of Agriculture, Washington D.C. 34p.
Monsen, S. B., R. Stevens, and N. L. Shaw. 2004. Grasses. Pages 295-424 in Restoring western ranges and wildlands, vol. 2. Gen. Tech. Rep. RMRS-GTR-136. USDA, Forest Service, Rocky Mountain Research Station, Fort Collins, CO.
Monsen, S. B., R. Stevens, S. C. Walker, and N. E. West. 1996. The competitive influence of seeded smooth brome (Bromus inermis) and intermediate wheatgrass (Thinopyron intermedium) within aspen-mountain brush communities of central Utah. In: West, N. E. (ed.), Rangelands in a Sustainable Biosphere: Proceedings of the Fifth International Rangeland Congress. 1995, July 23- 28. Salt Lake City, UT. Society for Range Management, Denver CO. Pages 379-380.
Morris, M. S., Shmautz, J. E., Stickney, P. F. 1962. Winter field key to the native shrubs of Montana. Bulletin 23. Bozeman, MT: Montana State University, Forest and Conservation Experiment Station; U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 70p.
Mueggler, W. F. 1989. Age distribution and reproduction of intermountain aspen stands. Western Journal of Applied Forestry 4(2):41-45.
Noste, N. V., and C. L. Bushey. 1987. Fire response of shrubs of dry forest habitat types in Montana and Idaho. Gen. Tech. Rep. INT-239. USDA, Forest Service, Intermountain Research Station, Ogden, UT. 22p.
Perala, D. A. 1990. Populus tremuloides. Michx. Quaking aspen. Pages 555-569 in R.M. Burns and B.H. Honkala (eds.). Silvics of North America. Volume 2. Hardwoods. USDA Forest Service Agric. Handbook 654, Washington, D.C. Accessed Dec 18, 2018.
Plummer, A., D. R., Christensen and S. B. Monsen. 1968. Restoring big game range in Utah. Publication No. 68-3. Utah Division of Fish and Game. Forest Service, U.S. Department of Agriculture, Federal Aid in Wildlife Restoration Funds.
183p.
Potter, D. A. 1998. Forested Communities of the Upper Montane in the Central and Southern Sierra Nevada. Gen. Tech. Rep. PSW-GTR-169. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture; 319 p.
Potter, D. A. 2005. Riparian community classification: west slope, central and southern Sierra Nevada, California. Technical Report R5-TP-022. USDA Forest Service, Pacific Southwest Research Station, Berkeley, CA.
Sampson, A. W. 1919. Effect of Grazing Upon Aspen Reproduction. Bulletin No 741. U.S. Department of Agriculture, Washington D.C. 29p.
Shepperd, W. D., and Smith, F. W. 1993. The role of near-surface lateral roots in the life cycle of aspen in the central Rocky Mountains. Forest Ecology and Management, 61(1-2):157-170.
Shepperd, W. D., P. C. Rogers, D. Burton, and D. L. Bartos. 2006. Ecology, biodiversity, management and restoration of the aspen in the Sierra Nevada. U.S. Department of Agriculture, U.S. Forest Service, Rocky Mountain Research Station.
Smith, D. S., Fettig, S. M., and Bowker, M. A. 2016. Elevated Rocky Mountain elk numbers prevent positive effects of fire on quaking aspen (Populus tremuloides) recruitment. Forest Ecology and Management 362:46-54.
Strand, E. K., L. A. Vierling, S. C. Bunting, P. E. Gessler. 2009a. Quantifying successional rates in western aspen woodlands: Current conditions, future predictions. Forest Ecology and Management 257(8):1705-1715.
Strand, E. K., Vierling, L. A. and Bunting, S. C., 2009b. A spatially explicit model to predict future landscape composition of aspen woodlands under various management scenarios. Ecological Modelling 220(2):175-191.
Stringham, T. K., P. Novak-Echenique, P. Blackburn, C. Coombs, D. Snyder, and A. Wartgow. 2015. Final Report for USDA Ecological Site Description State-and-Transition Models, Major Land Resource Area 28A and 28B Nevada. University of Nevada Reno, Nevada Agricultural Experiment Station Research Report 2015-01.
Sullivan, J. 2009. Corynorhinus townsendii: Townsend's big-eared bat. Online. Animal Diversity Web, Museum of Zoology, University of Michigan.
Tilley, D. J., D. Ogle, L. St. John, L. Holzworth, W. Crowder, and M. Majerus. 2004a. Mountain Brome. USDA NRCS plant guide. USDA NRCS Plant Materials Center. USDA NRCS Idaho State Office, Idaho. 5p.
Tilley, D. J., Ogle, D., St. John, L., Holzworth, L., Crowder, W., Majerus, M. 2004b. Slender Wheatgrass. USDA NRCS Plant Guide. USDA NRCS Plant Materials Center. USDA NRCS Idaho State Office, Idaho.
Turner, M. G., W. H. Romme, R. A. Reed, and Tuskan, G. A. 2003. Post-fire aspen seedling recruitment across the Yellowstone (USA) landscape. Landscape Ecology 18(2):127-140.
[USDA] United States Department of Agriculture. 1988. Range Plant Handbook (Reproduction of the 1937 edition). Dover Publications, Inc.: New York. 848 p.
Walker, S. C. 1993. Effects of cattle and big game on the secondary succession of aspen-conifer understory following fire. Thesis. Provo, UT: Brigham Young University. 44p.
Wall, T. G., R. F. Miller, and T. J. Svejcar. 2001. Juniper encroachment into aspen in the Northwest Great Basin. Journal of Range Management. 54(6):691-698.
Warner, R. M., and N. J. Czaplewski. 1984. Mammalian Species No. 224: Myotis volans. The American Society of Mammalogists. 4p.
Wildlife Action Plan Team 2012. Nevada Wildlife Action Plan. Reno, NV: Nevada Department of Wildlife. Available: http://www.ndow.org/Nevada_Wildlife/Conservation/Nevada_Wildlife_Action_Plan/ [Accessed 4/13/2018].
Wright, H. A. 1985. Effects of fire on grasses and forbs in sagebrush-grass communities. In: K. D. Sanders and J. Durham, (eds.). Rangeland Fire Effects; A Symposium. 1984, November 27-29. USDI-BLM, Boise, ID. Pages 12-21.
Young, R. P. 1983. Fire as a vegetation management tool in rangelands of the Intermountain region. In: Monsen, S.B. and
N. Shaw (eds). Managing Intermountain rangelands—improvement of range and wildlife habitats: Proceedings. 1981, September 15-17; Twin Falls, ID; 1982, June 22-24; Elko, NV. Gen. Tech. Rep. INT-157. Ogden, UT. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Pages 18-31.
Ziegenhagen, L. L. 2003. Shrub reestablishment following fire in the mountain big sagebrush (Artemisia tridentata Nutt. ssp. vaseyana (Rydb.) Beetle) alliance. M.S. Oregon State University.
Major Land Resource Area
MLRA 023X
Malheur High Plateau
Correlated Map Unit Components
21589692, 21589888, 21590071, 21589484, 21590718, 21590573, 21590318, 21604132, 21729854, 21589657, 21589664, 21589672, 21501143, 21500842, 21501122, 21590802, 21590798, 21501205, 21501396, 21589668, 21590033
Stage
Provisional
Contributors
T Stringham (UNR under contract with BLM)
DMP
Click on box and path labels to scroll to the respective text.