Ecological site group R023XY913NV
Shallow Rocky 8-14 PZ Black Sagebrush
Last updated: 06/03/2024
Accessed: 02/27/2026
Ecological site group description
Key Characteristics
- Site does not pond or flood
- Landform other than dunes
- Surface soils are not clayey
- Sites are shrub or grass dominated
- [Criteria]MAP >10"
- Soils is shallow to root restrictive layer
- Soils Aridisols
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
Physiography
This group is on plateaus at elevations between 4,500 and 6,000 feet. Slopes are 5 to 30 percent.
Climate
The climate is classified as Cold Semi-Arid in the Koppen Classification System.
The area receives 8 to 14 inches of annual precipitation as snow in the winter and rain in spring and fall. Summers are generally dry.
The frost-free period is 60 to 110 days. The mean annual air temperature is between 46 and 48 °F.
Soil features
The soils in this group generally have a root-restrictive layer within 20 inches. The textures are loamy-skeletal. Soils have limited water holding capacity.
Taxonomically, the soils are Aridisols. The soil temperature regime is mesic.
Common soil series in this group are Loomis and Shinnpeak.
Vegetation dynamics
Ecological Dynamics and Disturbance Response:
An ecological site is the product of all the environmental factors responsible for its development. Each site has a set of key characteristics that influence its resilience to disturbance and resistance to invasives. According to Caudle et al. (2013), key characteristics include:
1. Climate factors such as precipitation and temperature.
2. Topographic characteristics such as aspect, slope, elevation, and landform.
3. Hydrologic processes such as infiltration and runoff.
4. Soil characteristics such as depth, texture, structure, and organic matter.
5. Plant communities and their functional groups and productivity.
6. Natural disturbance (fire, herbivory, etc.) regime.
Biotic factors that influence resilience include site productivity, species composition and structure, and population regulation and regeneration (Chambers et al., 2013).
In the Great Basin, most of the annual precipitation is received during the winter and early spring. This continental semiarid climate regime favors the growth and development of deep-rooted shrubs and herbaceous cool-season plants using the C3 photosynthetic pathway (Comstock & Ehleringer, 1992). Winter precipitation and slow melting of snow results in deeper percolation of moisture into the soil profile. Herbaceous plants, more shallow-rooted than shrubs, grow earlier in the growing season and thrive on spring rains, while the deeper-rooted shrubs lag in phenological development because they draw from deeply infiltrating moisture from snowmelt the previous winter. Periodic drought regularly influences sagebrush ecosystems, and drought duration and severity have increased throughout the 20th century in much of the Intermountain West. Major shifts away from historical precipitation patterns have the greatest potential to alter ecosystem function and productivity. Species composition and productivity can be altered by the timing of precipitation and water availability within the soil profile (Bates et al., 2006).
The Great Basin sagebrush communities have high spatial and temporal variability in precipitation both among years and within growing seasons (MacMahon, 1980). Nutrient availability is typically low but increases with elevation and closely follows moisture availability. Disturbance changes resource uptake and increases nutrient availability, often to the benefit of non-native species; native species are often damaged and their ability to use resources is depressed for a time, but resource pools may increase from lack of use and/or the decomposition of dead plant material following disturbance (Whisenant, 1999; Miller et al., 2013). The invasion of sagebrush communities by cheatgrass (Bromus tectorum) has been linked to disturbances (fire, abusive grazing) that result in fluctuations in resources (Beckstead & Augspurger, 2004; Chambers et al., 2007; Johnson et al., 2011).
Black sagebrush (Artemisia nova) grows primarily on shallow soils that are well drained, gravelly, and often calcareous (Thatcher, 1959; Hironaka, 1963; Zamora & Tueller, 1973). Black sagebrush is generally long-lived, so it is not necessary for new individuals to recruit every year for perpetuation of the stand. Infrequent, large recruitment events and simultaneous low, continuous recruitment is the foundation of population maintenance (Noy-Meir, 1973). Survival of the seedlings depends on adequate moisture conditions.
Native insect outbreaks are also important drivers of ecosystem dynamics in sagebrush communities. Climate is generally believed to influence the timing of insect outbreaks, especially outbreaks of a sagebrush defoliator called Aroga moth (Aroga websteri). Aroga moth infestations occurred in the Great Basin in the 1960s, the early 1970s, and have been ongoing in Nevada since 2004 (Bentz et al., 2008). Thousands of acres of sagebrush have been impacted, including black sagebrush (Henry, 1961), with partial to complete die-off observed (Gates, 1964; Hall, 1965). Aroga moth can partially or entirely kill individual plants or entire stands of big sagebrush (Artemisia tridentata) (Furniss & Barr, 1975).
The perennial bunchgrasses that are co-dominant with the shrubs on these sites include bluebunch wheatgrass (Pseudoroegneria spicata) and Thurber’s needlegrass (Achnatherum thurberianum). Webber needlegrass (Achnatherum webberi), bottlebrush squirreltail (Elymus elymoides), needle and thread (Hesperostipa comata), and Sandberg bluegrass (Poa secunda) are other important grass species. Grasses generally have somewhat shallower root systems than the shrubs on these sites; root densities of grasses are often as high as or higher than those of shrubs in the upper 0.5 meters of the soil profile. The general differences in root depth distributions between grasses and shrubs result in resource partitioning in these shrub/grass systems.
The range and density of Utah juniper (Juniperus osterosperma) have increased since the middle of the 19th century (Tausch, 1999; Miller & Tausch, 2001). Causes for expansion of Utah juniper into sagebrush ecosystems include wildfire suppression, historic livestock grazing, and climate change (Bunting, 1994). Mean fire return intervals prior to European settlement in black sagebrush ecosystems were greater than 100 years. This was frequent enough to inhibit the encroachment of Utah juniper into these low-productivity sagebrush cover types (Kitchen & McArthur, 2007).Thus, trees were isolated to fire-safe areas such as rocky outcroppings and areas with low productivity. An increase in crown density causes a decrease in understory perennial vegetation and an increase in bare ground. This allows the invasion of non-native annual species such as cheatgrass. When annual species are present in the understory, wildfire can become more frequent and increase in intensity. With frequent wildfires, these plant communities can convert to annual plant communities with sprouting shrubs.
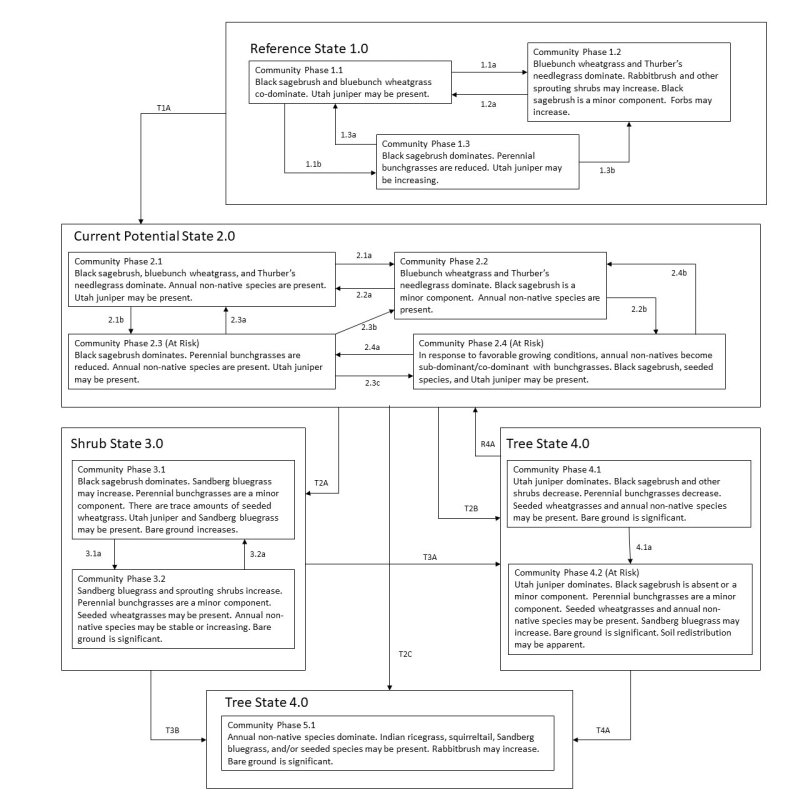
The ecological sites in this group have low to moderate resilience to disturbance and resistance to invasion. Resilience increases with elevation, northerly aspect, precipitation, and nutrient availability. Five possible states have been identified for this group.
Annual Invasive Grasses:
The species most likely to invade these sites are cheatgrass and medusahead (Taeniatherum). Both species are cool- season, annual grasses that maintain an advantage over native plants in part because they are prolific seed producers, able to germinate in the autumn or spring, tolerant of grazing, and increase with frequent fire (Klemmedson & Smith, 1964 Miller et al., 1999). Medusahead and cheatgrass originated from Eurasia and both were first reported in North America in the late 1800s (Mack & Pyke, 1983; Furbush, 1953). Pellant and Hall (1994) found 3.3 million acres of public lands dominated by cheatgrass and suggested that another 76 million acres were susceptible to invasion by winter annuals including cheatgrass and medusahead. By 2003, medusahead occupied approximately 2.3 million acres in 17 western states (Rice 2005). In the Intermountain West, the exponential increase in dominance by medusahead has largely been at the expense of cheatgrass (Harris, 1967; Hironaka, 1994). Medusahead matures 2 to 3 weeks later than cheatgrass (Harris, 1967) and recently, James et al. (2008) measured leaf biomass over the growing season and found that medusahead maintained vegetative growth later in the growing season than cheatgrass. Mangla et al. (2011) also found medusahead had a longer period of growth and more total biomass than cheatgrass and hypothesized this difference in relative growth rate may be due to the ability of medusahead to maintain water uptake as upper soils dry compared to co-occurring species, especially cheatgrass. Medusahead litter has a slow decomposition rate because of its high silica content, allowing it to accumulate over time and suppress competing vegetation (Bovey et al., 1961; Davies & Johnson, 2008). Harris (1967) reported medusahead roots have thicker cell walls compared to those of cheatgrass, allowing it to more effectively conduct water, even in very dry conditions.
Recent modeling and empirical work by Bradford and Lauenroth (2006) suggest that seasonal patterns of precipitation input and temperature are also key factors determining regional variation in the growth, seed production, and spread of invasive annual grasses. Collectively, the body of research suggests that the invasion and dominance of medusahead onto native grasslands and cheatgrass-infested grasslands will continue to increase in severity because conditions that favor native bunchgrasses or cheatgrass over medusahead are rare (Mangla et al., 2011). Medusahead replaces native vegetation and cheatgrass directly by competition and suppression; it replaces native vegetation indirectly by increasing fire frequency.
Methods to control medusahead and cheatgrass include herbicide, fire, grazing, and seeding of primarily non-native wheatgrasses. Mapping potential or current invasion vectors is a management method designed to increase the cost effectiveness of control methods. A study by Davies et al. (2013) found an increase in medusahead cover near roads. Cover was higher near animal trails than random transects but the difference was less evident. This implies that vehicles and animals aid the spread of the weed; however, vehicles are the major vector of movement. Spraying with herbicide (Imazapic or Imazapic + glyphosate) and seeding with crested wheatgrass (Agropyron cristatum) and Sandberg bluegrass have been more successful at combating medusahead and cheatgrass than spraying alone (Sheley et al., 2012). Where native bunchgrasses are missing from the site, revegetation of medusahead- or cheatgrass-invaded rangelands has shown a higher likelihood of success when using introduced perennial bunchgrasses such as crested wheatgrass (Davies et al., 2015). Butler et al. (2011) tested four herbicides (Imazapic, Imazapic + glyphosate, rimsulfuron, and sulfometuron + Chlorsulfuron), using herbicide-only treatments, for suppression of cheatgrass, medusahead, and ventenata (Ventenata dubia) within residual stands of native bunchgrass. Additionally, they tested the same four herbicides followed by seeding of six bunchgrasses (native and non-native) with varying success. Herbicide-only treatments appeared to remove competition for established bluebunch wheatgrass by providing 100 percent control of ventenata and medusahead and greater than 95 percent control of cheatgrass. However, caution in using these results is advised, as only one year of data was reported.
Prescribed fire has also been utilized in combination with the application of pre-emergent herbicide to control medusahead and cheatgrass (J. L. Vollmer & J. G. Vollmer, 2008). Mature medusahead or cheatgrass is very flammable and fire can be used to remove the thatch layer, consume standing vegetation, and even reduce seed levels. Furbush (1953) reported that timing a burn while the seeds were in the milk stage effectively reduced medusahead the following year. He further reported that adjacent unburned areas became a seed source for reinvasion the following year.
When considering the combination of pre-emergent herbicide and prescribed fire for invasive annual grass control, it is important to assess the tolerance of desirable brush species to the herbicide being applied. J. L. Vollmer and J. G. Vollmer (2008) tested the tolerance of mountain mahogany (Cercocarpus montanus), antelope bitterbrush (Purshia tridentata), and multiple sagebrush species to three rates of Imazapic and the same rates with methylated seed oil as a surfactant. They found a cheatgrass control program in an antelope bitterbrush community should not exceed Imazapic at 8 ounces per acre with or without surfactant. Sagebrush, regardless of species or rate of application, was not affected. However, many environmental variables were not reported in this study and managers should install test plots before broad scale herbicide application is initiated.
Fire Ecology:
Fire is not a major ecological component of these community types (Winward, 2001). Fire isinfrequent. Fire return intervals have been estimated at 100 to 200 years (Kitchen & McArthur, 2007). Fires were probably patchy and very infrequent due to the low productivity of these sites. Black sagebrush plants have no morphological adaptations for surviving fire and must reestablish from seed (Wright et al., 1979). The ability of black sagebrush to establish after fire mostly depends on the number of seeds deposited in the seed bank the year before the fire. Seeds typically do not persist in the soil for more than one growing season (Beetle, 1960). However, a few seeds may remain viable in the soil for two years (Meyer, 2008). Even in dry storage, black sagebrush seed viability drops rapidly over time, from 81 percent to 1 percent viability after 2 and 10 years of storage, respectively (Stevens et al., 1981). Thus, repeated, frequent fires can eliminate black sagebrush from a site, although black sagebrush in zones that receive 12 to 16 inches of annual precipitation have been found to have greater fire survival (Boltz, 1994). In lower precipitation zones, rabbitbrush (Chrysothamnus sp.) may become the dominant shrub species following fire, often with an understory of Sandberg bluegrass and/or cheatgrass and other weedy species.
The effect of fire on bunchgrasses relates to culm density, culm-leaf morphology, and the size of the plant. The initial condition of bunchgrasses on a site and seasonality and intensity of the fire all factor into the individual species response. For most forbs and grasses, the growing points are located at or below the soil surface. This provides relative protection from disturbances that decrease aboveground biomass, such as grazing or fire. Thus, fire mortality is more correlated to the duration and intensity of heat, which is related to culm density, culm-leaf morphology, size of plant, and abundance of old growth (Wright, 1971; Young, 1983).
Fire will remove aboveground biomass from bluebunch wheatgrass, but plant mortality is generally low (Robberecht & Defossé, 1995) because the buds are underground (Conrad & Poulton, 1966) or protected by foliage. Uresk et al. (1976) reported burning increased vegetative and reproductive vigor of bluebunch wheatgrass. Thus, bluebunch wheatgrass experiences slight damage from fire but is more susceptible to fire damage in drought years (Young, 1983). Plant response varies depending on season, fire severity, fire intensity, and post-fire soil moisture availability.
Burning has been found to decrease the vegetative and reproductive vigor of Thurber’s needlegrass (Uresk et al., 1976). Fire can cause high mortality, in addition to reducing basal area and yield of Thurber’s needlegrass (Britton et al., 1990). The fine leaves and densely tufted growth form make this grass susceptible to subsurface charring of the crowns (Wright & Klemmedson, 1965). Although timing of fire highly influences the response and mortality of Thurber’s needlegrass, smaller bunch sizes are less likely to be damaged by fire (Wright & Klemmedson, 1965). Thurber’s needlegrass often survives fire and continues growth or regenerates from tillers when conditions are favorable (Koniak, 1985; Britton et al., 1990). Reestablishment on burned sites is relatively slow due to low germination and competitive ability (Koniak, 1985). Cheatgrass is a highly successful competitor with seedlings of this needlegrass and may preclude reestablishment (Evans & Young, 1978).
Utah juniper is usually killed by fire and is most vulnerable to fire when it is under 4 feet tall (Bradley et al., 1992). Larger trees, because they have foliage farther from the ground and thicker bark, can survive low-severity fires, but when 60 percent or more of the crown is scorched, mortality occurs. Due to the low production of the understory vegetation, high-severity fires within this plant community are not likely and rarely became crown fires (Bradley et al., 1992; Miller & Tausch, 2001). Tree density on these sites increases with management that focuses on fire suppression, and with grazing management that favors the removal of fine fuels. Fire severity is likely to increase when cheatgrass increases in the understory. Utah juniper reestablishes from seed from nearby seed sources or surviving seeds. Utah juniper begins to produce seed at about 30 years of age (Bradley et al., 1992). Seeds establish best with a nurse plant such as sagebrush and rabbitbrush (Everett & Ward, 1984; Tausch & West, 1988; Bradley et al., 1992). Utah juniper woodlands reach maturity 85 to 150 years after fire (Barney & Frischknecht, 1974; Tausch & West, 1988).
The grasses likely to invade this site are cheatgrass and medusahead. These invasive grasses displace desirable perennial grasses, reduce livestock forage, and accumulate large fuel loads that foster frequent fires (Davies & Svejcar, 2008). Invasion by annual grasses can alter the fire cycle by increasing fire size, fire season length, rate of spread, numbers of individual fires, and likelihood of fires spreading into native or managed ecosystems (D’Antonio & Vitousek, 1992; Brooks et al., 2004). While historical fire return intervals are estimated at 15 to 100 years, areas dominated by cheatgrass are estimated to have a fire return interval of 3 to 5 years (Whisenant, 1990). The mechanisms by which invasive annual grasses alter fire regimes likely interact with climate. For example, cheatgrass cover and biomass vary with climate (Chambers et al., 2007) and are promoted by wet and warm conditions during the fall and spring. Invasive annual species can take advantage of high nitrogen availability following fire because of their higher growth rates and increased seedling establishment relative to native perennial grasses (Monaco et al., 2003).
Livestock/Wildlife Grazing Interpretations:
Black sagebrush palatability, depending on ungulate and season of use, is moderate to high (Horton, 1989; Wambolt, 1996). The palatability of black sagebrush increases the potential for negative impacts on remaining black sagebrush plants from grazing or browsing pressure following fire (Wambolt, 1996). Pronghorn utilize black sagebrush heavily (Beale & Smith, 1970). In a study by Beale and Smith (1970) on the Desert Experiment Range, black sagebrush comprised 68 percent of the pronghorn diet even though it was only the third most common plant, and fawns preferred black sagebrush, utilizing it more than all other forage species combined. Domestic livestock will also utilize black sagebrush. The domestic sheep industry that emerged in the Great Basin in the early 1900s was largely based on wintering domestic sheep in black sagebrush communities (Mozingo, 1987). Domestic sheep will browse black sagebrush during all seasons of the year depending on the availability of other forage species. They consume greater amounts in fall and winter. Black sagebrush is generally less palatable to cattle than to domestic sheep and wild ungulates (McArthur et al., 1979). However, cattle use of black sagebrush has also been shown to be greatest in fall and winter (Schultz & McAdoo, 2002), with only trace amounts being consumed in summer (Van Vuren, 1984).
Bluebunch wheatgrass is moderately grazing-tolerant and is very sensitive to defoliation during the active growth period (Blaisdell & Pechanec, 1949; Laycock, 1967; Anderson & Scherzinger, 1975). In studies, herbage and flower stalk production were reduced with clipping at all times during the growing season; clipping was most harmful, however, during the boot stage (Blaisdell & Pechanec, 1949; Britton et al., 1990) Tiller production and growth of bluebunch wheatgrass can be greatly reduced when clipping is coupled with drought (Busso & Richards, 1995). Mueggler (1975) estimated that low-vigor bluebunch wheatgrass may need up to 8 years rest to recover. Although an important forage species, it is not always the preferred species by livestock and wildlife.
Thurber's needlegrass is an important forage source for livestock and wildlife in the arid regions of the West (Ganskopp, 1988). The seeds are apparently not injurious, but grazing animals avoid them when the seeds begin to mature. Sheep, however, have been observed grazing the leaves closely, leaving stems untouched (Eckert & Spencer, 1987). Heavy grazing during the growing season has been shown to reduce the basal area of Thurber’s needlegrass (Eckert & Spencer, 1987). This suggests that both seasonality and utilization are important factors in management of this plant. A single defoliation, particularly during the boot stage, can reduce herbage production and root mass, thus potentially lowering the competitive ability of this needlegrass (Ganskopp, 1988).
Reduced bunchgrass vigor or density provides an opportunity for Sandberg bluegrass expansion and/or cheatgrass and other invasive species to occupy interspaces. This leads to increased fire frequency and potentially an annual plant community. Sandberg bluegrass increases under grazing pressure (Tisdale & Hironaka, 1981) and is capable of co-existing with cheatgrass. Excessive sheep grazing favors Sandberg bluegrass. Where cattle are the dominant grazers, cheatgrass often dominates (Daubenmire, 1970). Thus, depending on the season of use, the type of grazing animal, and site conditions, either Sandberg bluegrass or cheatgrass may become the dominant understory species with inappropriate grazing management.
Long-term disturbance response may be influenced by small differences in landscape topography. Concave areas hold more moisture and may retain deep-rooted perennial grasses, whereas convex areas are slightly less resilient and may have more Sandberg bluegrass present.
Inappropriate grazing practices can be tied to the success of medusahead, but eliminating grazing will not eradicate medusahead if it is already present (Wagner et al., 2001). Sheley and Svejcar (2009) reported that even moderate defoliation of bluebunch wheatgrass resulted in increased medusahead density. They suggested that disturbances such as plant defoliation limit soil resource capture, which creates an opportunity for exploitation by medusahead. Avoidance of medusahead by grazing animals allows medusahead populations to expand. This creates seed reserves that can infest adjoining areas and cause changes to the fire regime. Medusahead replaces native vegetation and cheatgrass directly by competition and suppression; it replaces native vegetation indirectly by increasing fire frequency.
Medusahead litter has a slow decomposition rate because of its high silica content, allowing it to accumulate over time and suppress competing vegetation (Bovey et al., 1961; Davies & Johnson, 2008).
References:
Bentz, B., D. Alston, and T. Evans. 2008. Great Basin Insect Outbreaks. In: J. Chambers, N. Devoe, A. Evenden (eds.). Collaborative Management and Research in the Great Basin -- Examining the issues and developing a framework for action Gen. Tech. Rep. RMRS-GTR-204. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, CO. Pages 45-48.
Beetle, A. A. 1960. A Study of Sagebrush: The Section Tridentatae of Artemisia. Wyoming Agricultural State Bulletin 368:1-83.
Beckstead, J., and Augspurger, C. K. 2004. An experimental test of resistance to cheatgrass invasion: limiting resources at different life stages. Biological Invasions 6(4):417-432.
Blaisdell, J. P. and J. F. Pechanec. 1949. Effects of Herbage Removal at Various Dates on Vigor of Bluebunch Wheatgrass and Arrowleaf Balsamroot. Ecology 30(3):298-305.
Boltz, M. 1994. Factors influencing postfire sagebrush regeneration in south-central Idaho. In: S. B. Monsen and S. G. Kitchen, (eds.). Proceedings-ecology and management of annual rangelands. Gen. Tech. Rep. INT-GTR-313. 1992, May 18-21. Boise, ID. USDA Forest Service, Intermountain Research Station, Ogden, UT. Pages 281-290.
Bovey, W. R., D. Le Tourneau, and C. L. Erickson. 1961. The chemical composition of medusahead and downy brome. Weeds 9(2):307-311.
Bradford, J. B., and W. K. Lauenroth. 2006. Controls over invasion of Bromus tectorum: The importance of climate, soil, disturbance and seed availability. Journal of Vegetation Science 17(6):693-704.
Bradley, A. F., N. V. Noste, and W. C. Fischer. 1992. Fire ecology of forests and woodlands in Utah. Gen. Tech. Rep. INT-287. U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 128 p.
Britton, C. M., G. R. McPherson, and F. A. Sneva. 1990. Effects of burning and clipping on five bunchgrasses in eastern Oregon. Great Basin Naturalist 50(2):115-120.
Brooks, M. L., C. M. D'Antonio, D. M. Richardson, J. B. Grace, J. E. Keeley, J. M. Ditomaso, R. J. Hobbs, M. Pellant, and D. Pyke. 2004. Effects of Invasive Alien Plants on Fire Regimes. BioScience 54(7):677-688.
Bunting, S. 1994. Effects of Fire on Juniper woodland ecosystems in the Great Basin. In: S. B. Monsen and S. G. Ketchum, (eds.). Proceedings: Ecology and Management of Annual Rangelands. Gen. Tech. Rep. INT-GTR-313. 1992, May 18-22. U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Boise, ID. Pages 53-55.
Butler, M., R. Simmons, and F. Brummer. 2011. Restoring Central Oregon Rangeland from Ventenata and Medusahead to a Sustainable Bunchgrass Environment – Warm Springs and Ashwood. Central Oregon Agriculture Research and Extension Center. COARC 2010. Pages 77-82.
Comstock, J. P. and J. R. Ehleringer. 1992. Plant adaptation in the Great Basin and Colorado Plateau. Western North American Naturalist 52(3):195-215.
D'Antonio, C. M., and P. M. Vitousek. 1992. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review of Ecology and Systematics 23:63-87.
Daubenmire, R. 1970. Steppe vegetation of Washington. Technical Bulletin 62. Washington Agriculture Experiment Station. 131 p.
Davies, K. W., and D. D. Johnson. 2008. Managing medusahead in the intermountain west is at a critical threshold. Rangelands 30(4):13-15.
Davies, K. W., and T. J. Svejcar. 2008. Comparison of medusahead-invaded and noninvaded Wyoming big sagebrush steppe in southeastern Oregon. Rangeland Ecology and Management 61(6):623-629.
Davies, K. W., A. M. Nafus, and M. D. Madsen. 2013. Medusahead invasion along unimproved roads, animal trails, and random transects. Western North American Naturalist 73(1):54-59.
Davies, K. W., C. S. Boyd, D. D. Johnson, A. M. Nafus, and M. D. Madsen. 2015. Success of seeding native compared with introduced perennial vegetation for revegetating medusahead-invaded sagebrush rangeland. Rangeland Ecology & Management 68(3):224-230.
Everett, R. L. and K. Ward. 1984. Early plant succession on pinyon-juniper controlled burns. Northwest Science 58(1):57-68.
Furbush, P. 1953. Control of Medusa-Head on California Ranges. Journal of Forestry 51(2):118-121.
Furniss, M. M. and W. F. Barr. 1975. Insects affecting important native shrubs of the northwestern United States Gen. Tech. Rep. INT-19. Intermountain Forest and Range Experiment Station, U.S. Department of Agriculture, Forest Service. Ogden, UT. 68 p.
Hall, R. C. 1965. Sagebrush defoliator outbreak in Northern California. Res. Note PSW-RN-075. U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station, Berkeley, CA. 12 p.
Harris, G. A. 1967. Some Competitive Relationships between Agropyron spicatum and Bromus tectorum. Ecological Monographs 37(2):89-111.
Henry, J. E. 1961. Biology of the sagebrush defoliator Aroga websteri Clarke in Idaho. M.S. Thesis. University of Idaho, Moscow, Idaho.
Hironaka, M. 1963. Plant environment relations of major species in sagebrush-grass vegetation of southern Idaho. Thesis. Ph. D. Diss. University of Wisconsin, Madison.
Hironaka, M. 1994. Medusahead: Natural Successor to the Cheatgrass Type in the Northern Great Basin. In: Proceedings of Ecology and Management of Annual Rangelands. USDA Forest Service, Intermountain Research Station. Gen. Tech. Rep. INT-GTR-313. Pages 89-91.
Horton, H. 1989. Interagency forage and conservation planting guide for Utah. Extension Circular 433. Utah State University, Utah Cooperative Extension Service, Logan UT.
James, J., K. Davies, R. Sheley, and Z. Aanderud. 2008. Linking nitrogen partitioning and species abundance to invasion resistance in the Great Basin. Oecologia 156(3):637-648.
Johnson, B. G.; Johnson, D. W.; Chambers, J. C.; Blank, B.R. 2011. Fire effects on the mobilization and uptake of nitrogen by cheatgrass (Bromus tectorum L.). Plant and Soil 341(1-2):437-445.
Kitchen, S. G. and E. D. McArthur. 2007. Big and black sagebrush landscapes. In: S. Hood, M. Miller (eds.). Fire ecology and mangement of the major ecosystems of southern Utah. Gen. Tech. Rep. RMRS-GTR-202. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, CO. Pages 73-95.
Klemmedson, J. O., and J. G. Smith. 1964. Cheatgrass (Bromus Tectorum L.). The botanical review 30(2):226-262.
Koniak, S. 1985. Succession in pinyon-juniper woodlands following wildfire in the Great Basin. The Great Basin Naturalist 45(3):556-566.
Mack, R. N., and D. Pyke. 1983. The Demography of Bromus Tectorum: Variation in Time and Space. Journal of Ecology 71(1):69-93.
MacMahon, J. A. 1980. Ecosystems over time: succession and other types of change. In: Waring, R., ed. Proceedings—Forests: fresh perspectives from ecosystem analyses. Biological Colloquium. Corvallis, OR: Oregon State University. Pages 27-58.
Mangla, S., R. Sheley, and J. J. James. 2011. Field growth comparisons of invasive alien annual and native perennial grasses in monocultures. Journal of Arid Environments 75(2):206-210.
McArthur, E. D., A. C. Blauer, A. P. Plummer, and R. Stevens. 1979. Characteristics and hybridization of important Intermountain shrubs. III. Sunflower family. Res. Pap. INT-220. Ogden, UT. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 82 p.
Meyer, S. E. 2008. Artemisia L.: Sagebrush. Pages 274-280 in F. T. Bonner and R. P. Karrfalt, editors. The Woody Plant Seed Manual. Agriculture Handbook No. 727. U.S. Department of Agriculture, Forest Service, Washington, DC.
Miller, H. C., Clausnitzer, D., and Borman, M. M. 1999. Medusahead. In: R. L. Sheley and J. K. Petroff (eds.). Biology and Management of Noxious Rangeland Weeds. Corvallis, OR: Oregon State University Press. Pages 272-281.
Miller, R. F., and Tausch, R. J. 2001. The Role of Fire in Juniper and Pinyon Woodlands: A Descriptive Analysis. In: Galley. K. M. and T. P. Wilson (eds.), Fire Conference 2000: The First National Congress on Fire Ecology, Prevention, and Management; Tallahassee, FL: Tall Timbers Research Station, San Diego, CA, USA. Pages 15-30.
Miller, R. F., Chambers, J. C., Pyke, D. A., Pierson, F. B. and Williams, C. J., 2013. A review of fire effects on vegetation and soils in the Great Basin Region: response and ecological site characteristics. Gen. Tech. Rep. RMRS-GTR-308. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. 126p.
Monaco, T. A., Charles T. Mackown, Douglas A. Johnson, Thomas A. Jones, Jeanette M. Norton, Jay B. Norton, and Margaret G. Redinbaugh. 2003. Nitrogen effects on seed germination and seedling growth. Journal of Range Management 56(6):646-653.
Mozingo, H. N. 1987. Shrubs of the Great Basin: A Natural History. Pages 67-72 in H. N. Mozingo, editor. Shrubs of the Great Basin. University of Nevada Press, Reno, NV.
Pellant, M., and C. Hall. 1994. Distribution of two exotic grasses in intermountain rangelands: status in 1992. USDA Forest Service Gen. Tech. Rep. INT-GTR-313S. Pages 109-112.
Rice, P. M. 2005. Medusahead (Taeniatherum caput-medusae (L.) Nevski). In: C. L. Duncan and J. K. Clark (eds.). Invasive plants of range and wildlands and their environmental, economic, and societal impacts. Weed Science Society of America, Lawrence, KS.
Schultz, B. W., and J. K. McAdoo. 2002. Common Sagebrush in Nevada. Special Publication SP-02-02. University of Nevada, Cooperative Extension, Reno, NV. 9 p.
Sheley, R. L., Svejcar T. J. 2009. Response of bluebunch wheatgrass and medusahead to defoliation. Rangeland Ecology & Management 62(3):278-283.
Sheley, R. L., E. A. Vasquez, A. Chamberlain, and B. S. Smith. 2012. Landscape-scale rehabilitation of medusahead (Taeniatherum caput-medusae)-dominated sagebrush steppe. Invasive Plant Science and Management 5(4):436-442.
Stevens, R., K. R. Jorgensen, and J. N. Davis. 1981. Viability of seed from thirty-two shrub and forb species through fifteen years of warehouse storage. Western North American Naturalist 41(3):274-277.
Tausch, R. J. 1999. Historic pinyon and juniper woodland development. In: S. B. Monsen and R. Stevens, (comps.). Proceedings: Ecology and management of pinyon-juniper communities within the Interior West. 1997, September 15-18. Provo, UT. Proceedings RMRS-P-9. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Provo, UT. Pages 12-19.
Thatcher, A. P. 1959. Distribution of sagebrush as related to site differences in Albany County, Wyoming. Journal of Range Management 12(2):55-61.
Tisdale, E. W. and M. Hironaka. 1981. The sagebrush-grass region: A review of the ecological literature. Bulletin 33. University of Idaho, Forest, Wildlife and Range Experiment Station. Moscow, ID. 31p.
Vollmer, J. L., and J. G. Vollmer. 2008. Controlling cheatgrass in winter range to restore habitat and endemic fire United States Department of Agriculture, Forest Service. RMRS-P-52. Pages 57-60.
Wagner, J. A., R. E. Delmas, J. A. Young. 2001. 30 years of medusahead: return to fly blown-flat. Rangelands 23(3):6-9.
Whisenant, S., 1999. Repairing Damaged Wildlands: a process-orientated, landscape-scale approach (Vol. 1). Cambridge, UK: Cambridge University Press. 312p.
Winward, A. H. 2001. Sagebrush taxonomy and ecology workshop. In: Vegetation, wildlife and fish ecology and rare species management - Wasatch-Cache National Forest. U.S. Department of Agriculture, Forest Service, Intermountain Region, Uinta-Wasatch-Cache National Forest, Logan, UT.
Wright, H. A., L. F. Neuenschwander, and C. M. Britton. 1979. The role and use of fire in sagebrush-grass and pinyon-juniper plant communities: A state-of-the-art review. Gen. Tech. Rep. INT-58. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 48p.
Young, R. P. 1983. Fire as a vegetation management tool in rangelands of the Intermountain region. In: Monsen, S.B. and N. Shaw (eds). Managing Intermountain rangelands—improvement of range and wildlife habitats: Proceedings. 1981, September 15-17; Twin Falls, ID; 1982, June 22-24; Elko, NV. Gen. Tech. Rep. INT-157. Ogden, UT. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Pages 18-31.
Zamora, B., and P. T. Tueller. 1973. Artemisia arbuscula, A. longiloba, and A. nova habitat types in northern Nevada. Great Basin Naturalist 33(4):225-242.
Major Land Resource Area
MLRA 023X
Malheur High Plateau
Correlated Map Unit Components
21659724, 21660139, 21660144, 21660149, 21659874, 21660240, 21500642
Stage
Provisional
Contributors
T Stringham (UNR under contract with BLM)
DMP
Click on box and path labels to scroll to the respective text.