Ecological site group R023XY916NV
Seasonally Flooded Basin Wildrye
Last updated: 06/03/2024
Accessed: 02/08/2026
Ecological site group description
Key Characteristics
- Site subject to Ponding or Flooding
- Site is not sodic
- Soils are loamy
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
Physiography
This group is on basins at elevations between 4,000 and 6,500 feet. Slopes are less than 5 percent. 1 percent slope is the most typical.
The low-lying sites of this group receive additional water as run in from adjacent uplands. The water table is at or near the surface during the growing season.
Climate
The climate is classified as Cold Semi-Arid in the Koppen Classification System.
The area receives 8 to 14 inches of annual precipitation as snow in the winter and rain in the spring and fall. Summers are generally dry.
The frost-free period is 60 to 120 days.
Soil features
The soils in this group have ashy or clayey textures and are very deep. The soil temperature regime is either mesic or frigid.
Taxonomically, the soils are either Aridisols or Mollisols.
Common soil series in this group include Updike, Crutcher, Smocreek, Dugway, and Jesayno.
Vegetation dynamics
Ecological Dynamics and Disturbance Response:
An ecological site is the product of all the environmental factors responsible for its development. Each site has a set of key characteristics that influence its resilience to disturbance and resistance to invasives. According to Caudle et al. (2013), key characteristics include:
1. Climate factors such as precipitation and temperature.
2. Topographic characteristics such as aspect, slope, elevation, and landform.
3. Hydrologic processes such as infiltration and runoff.
4. Soil characteristics such as depth, texture, structure, and organic matter.
5. Plant communities and their associated functional groups and productivity.
6. Natural disturbance (fire, herbivory, etc.) regime.
Biotic factors that that influence resilience include site productivity, species composition and structure, and population regulation and regeneration (Chambers et al., 2013).
The ecological sites in this group are dominated by deep-rooted, cool-season, perennial bunchgrasses and long-lived shrubs (at least 50 years old) with high root to shoot ratios. The dominant shrubs usually root to the full depth of the winter-spring soil moisture recharge, which ranges from 1.0 to over 3.0 meters (Dobrowolski et al., 1990). Root length of mature sagebrush plants was measured to a depth of 2 meters in alluvial soils in Utah (Richards & Caldwell, 1987). These shrubs have a flexible generalized root system with development of both deep taproots and laterals near the surface (Comstock & Ehleringer, 1992).
The perennial bunchgrasses on these sites generally have somewhat shallower root systems than the shrubs; root densities of the grasses are often as high as or higher than those of shrubs in the upper 0.5 meters but densities taper off more rapidly than shrubs. However, basin wildrye (Leymus cinereus) is weakly rhizomatous and has been found to root to depths of 1 meter or more and exhibits greater lateral root spread than many other grass species (Abbott et al., 1991). The general differences in root depth distributions between grasses and shrubs result in resource partitioning in these shrub/grass systems.
In the Great Basin, most of the annual precipitation is received during the winter and early spring. This continental semiarid climate regime favors the growth and development of deep-rooted shrubs and herbaceous, cool-season plants using the C3 photosynthetic pathway (Comstock & Ehleringer, 1992). Winter precipitation and slow melting of snow results in deeper percolation of moisture into the soil profile. Herbaceous plants, more shallow-rooted than shrubs, grow earlier in the growing season and thrive on spring rains, while the deeper-rooted shrubs lag in phenological development because they draw from deeply infiltrating moisture from snowmelt the previous winter. Periodic drought regularly influences sagebrush ecosystems, and drought duration and severity have increased throughout the 20th century in much of the Intermountain West. Major shifts away from historical precipitation patterns have the greatest potential to alter ecosystem function and productivity. Species composition and productivity can be altered by the timing of precipitation and water availability within the soil profile (Bates et al., 2006).
The Great Basin sagebrush communities have high spatial and temporal variability in precipitation both among years and within growing seasons (MacMahon, 1980). Nutrient availability is typically low but increases with elevation and closely follows moisture availability. Disturbance changes resource uptake and increases nutrient availability, often to the benefit of non-native species; native species are often damaged and their ability to use resources is depressed for a time, but resource pools may increase from lack of use and/or the decomposition of dead plant material following disturbance (Whisenant, 1999; Miller et al., 2013). The invasion of sagebrush communities by cheatgrass (Bromus tectorum) has been linked to disturbances (fire, abusive grazing) that result in fluctuations in resources (Beckstead & Augspurger, 2004; Chambers et al., 2007; Johnson et al., 2011). One of the primary disturbances on these ecological sites is channel incision that lowers the seasonal water table and facilitates an increase in shrubs and a decrease in perennial bunchgrasses (Chambers et al., 2004). With continued site degradation, rubber rabbitbrush (Ericameria nauseosa) becomes the dominant plant.
Basin wildrye is a large, cool-season, perennial bunchgrass with an extensive, deep, coarse, fibrous, weakly rhizomatous root system (Reynolds & Fraley, 1989; Zschaechner, 1985). Clumps may reach up to 6 feet in height (Ogle et al., 2012). Basin wildrye does not tolerate long periods of inundation; it prefers cycles of wet winters and dry summers and is most common on deep soils with high water holding capacities or seasonally high water tables (Ogle et al., 2012; Perryman & Skinner, 2007).
Although no longer considered a different species than Sandberg bluegrass (Poa secunda), Nevada bluegrass occupies a different ecological niche and is not as tolerant of grazing as Sandberg bluegrass. The species occupies an unusually wide elevational range; it grows anywhere from a few hundred feet above sea level to near 11,000 feet in Colorado. It often grows on partially shaded stream banks and creek bottoms, irrigated fields, and meadows, and where moisture is sufficient the stand can produce a hay crop (USDA, 1988).
Native insect outbreaks are also important drivers of ecosystem dynamics in sagebrush communities. Climate is generally believed to influence the timing of insect outbreaks, especially outbreaks of a sagebrush defoliator called Aroga moth (Aroga websteri). Aroga moth infestations occurred in the Great Basin in the 1960s, the early 1970s, and have been ongoing in Nevada since 2004 (Bentz et al., 2008). Thousands of acres of big sagebrush (Artemisia tridentata) have been impacted, with partial to complete die-off observed. Aroga moth can partially or entirely kill individual plants or entire stands of big sagebrush (Furniss & Barr, 1975).
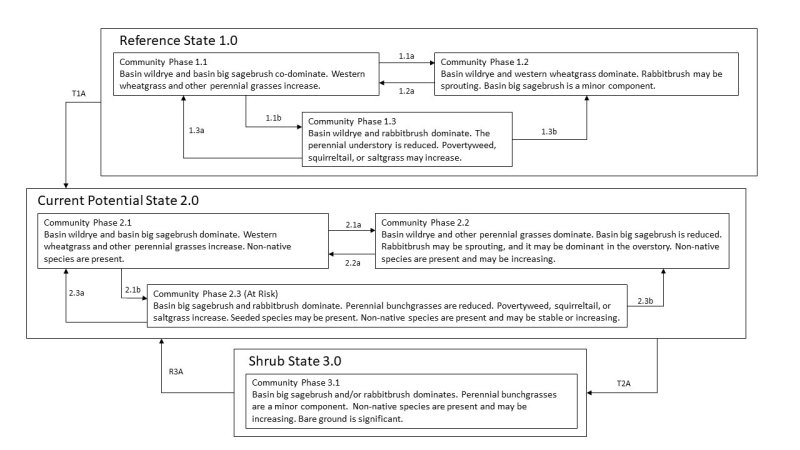
The ecological sites in this group have moderate resilience to disturbance and resistance to invasion. The primary disturbances on these ecological sites are drought, fire, flooding, Aroga moth infestation, and channel incision or other disturbance leading to a lowered seasonal water table. Disturbance facilitates an increase in shrubs and a decrease in basin wildrye. Troublesome non-native weeds such as broadleaved pepperweed (Lepidium latifolium), whitetop (Cardaria draba), Scotch cottonthistle (Onopordum acanthium), or bull thistle (Cirsium vulgare) are potential invaders on these sites. Three possible states have been identified for this group.
Hydrology:
The typical seasonally high water table occurs at depths within 60 inches of the surface. This allows for significant production of basin wildrye. Seasonally high water tables are necessary for maintenance of site productivity and reestablishment of basin wildrye stands following disturbances such as fire, drought or excessive herbivory (Eckert et al., 1973). The sensitivity of basin wildrye seedling establishment to reduced soil water availability increases as soil pH increases (Stuart et al., 1971). Lowering of the water table by extended drought, channel incision, or water pumping will decrease basin wildrye production and establishment while sagebrush, greasewood (Sarcobatus vermiculatus), rabbitbrush, and invasive weeds increase. Farming and abandonment may facilitate the creation of a surface vesicular crust, increase surface ponding, and decrease infiltration, which in turn lead to dominance by sprouting shrubs with a weedy understory.
In many areas, the sites of this group develop where a channel becomes entrenched, consequently lowering the water table required to support a meadow plant community. Further channel incisement and associated water table lowering cause site degradation. Most Great Basin streams have been prone to incision for the past two thousand years, so separating changes attributable to ongoing stream incision from those caused by humans can be difficult (Chambers et al., 2004). The most direct evidence that anthropogenic disturbance attributes to stream incision in the central Great Basin is derived from research on the effects of roads on riparian areas (Forman & Deblinger, 2000; Trombulak & Frissel, 2000).
Assigning cause and effect to more diffuse disturbances such as livestock grazing is more difficult. In general, overuse of riparian areas by livestock can negatively affect stream bank and channel stability. Localized changes in stream morphology have been associated with heavy livestock use in the western United States (Trimble & Mendle, 1995; Belsky et al., 1999). However, data that clearly demonstrate the relationship between regional stream incision and overuse by livestock have not been collected for the Great Basin (Chambers et al., 2004). The impact of feral horse use on riparian systems is also in need of documentation. Regarding restoration and management, it is important to recognize that particular streams have a greater sensitivity to both natural and management disturbances. For further guidance, see Chambers et al. (2004), Rosgen (2006), or USDA-NRCS (1998).
Fire Ecology:
The effect of fire on bunchgrasses relates to culm density, culm-leaf morphology, and the size of the plant. The initial condition of bunchgrasses on a site and seasonality and intensity of the fire all factor into the individual species response. For most forbs and grasses, the growing points are located at or below the soil surface. This provides relative protection from disturbances that decrease aboveground biomass, such as grazing or fire. Thus, fire mortality is more correlated to duration and intensity of heat, which is related to culm density, culm-leaf morphology, size of plant, and abundance of old growth (Wright, 1971; Young, 1983).
Basin wildrye is relatively resistant to fire, particularly fire during the dormant season, as plants sprout from surviving root crowns and rhizomes (Zschaechner, 1985). Miller et al. (2013) reported increased total shoot and reproductive shoot densities in the first year following fire, although by year two there was little difference between burned and control treatments.
In many basin big sagebrush (Artemisia tridentata ssp. tridentata) communities, changes in fire frequency co-occurred with fire suppression, livestock grazing, and off-highway vehicle (OHV) use. Few, if any, fire history studies have been conducted on basin big sagebrush. However, Sapsis and Kauffman (1991) suggest that fire return intervals in basin big sagebrush communities are intermediate between mountain big sagebrush (Artemisia tridentata ssp. vaseyana), 15 to 25 years, and Wyoming big sagebrush (Artemisia tridentata ssp. wyomingensis), 50 to 100 years. Fire severity in big sagebrush communities is "variable" depending on weather, fuels, and topography. However, fire in basin big sagebrush communities is typically stand-replacing (Sapsis & Kauffman, 1991). Basin big sagebrush does not sprout after fire. Because of the time needed to produce seed, it is eliminated by frequent fires (Bunting et al., 1987). Basin big sagebrush reestablishes on a site primarily from off-site seed or seed from plants that survive in unburned patches.
Approximately 90 percent of big sagebrush seed is dispersed within 30 feet (9 meters) of the parent shrub (Goodrich et al., 1985). The maximum seed dispersal is approximately 108 feet (33 meters) from the parent shrub (Shumar & Anderson, 1986). Therefore, regeneration of basin big sagebrush after stand-replacing fires is difficult and depends upon proximity of residual mature plants and favorable moisture conditions (Johnson & Payne, 1968; Humphrey, 1984).
The majority of research concerning rabbitbrush has been conducted on green rabbitbrush (Ericameria teretifolia). Green rabbitbrush has a large taproot and is known to be shorter-lived and less competitive than sagebrush. Seedling density, flower production, and shoot growth decline as competition from other species increases (McKell & Chilcote, 1957; Miller et al., 2013; Young & Evans, 1974). Depending on fire severity, rabbitbrush may increase after fire. Rubber rabbitbrush is top-killed by fire, but can resprout after fire and can also establish from seed (Young, 1983). Shortened fire intervals within this ecological site group favor a beardless wildrye (Leymus triticoides) understory with varying cover of rabbitbrush-dominated overstory.
Hydrologic modification of the sites of this group may be caused by channel incision or gully formation during post-fire rain events. Channel incision or gully formation can lower a site’s water table, which in turn dries out the site and favors the dominance of sagebrush and rabbitbrush over the herbaceous component.
Livestock/Wildlife Grazing Interpretations:
Basin wildrye is valuable forage for livestock (Ganskopp et al., 2007) and wildlife. It is intolerant of heavy, repeated, or spring grazing (Krall et al., 1971). Basin wildrye is used often as a winter feed for livestock and wildlife since it not only provides roughage above the snow but also cover in the early spring months (Majerus, 1992).
Overgrazing leads to an increase in big sagebrush, rabbitbrush, and greasewood; it leads to a decline in understory plants like basin wildrye and Nevada bluegrass. Reduced bunchgrass vigor or density provides an opportunity for other species—beardless wildrye, saltgrass (Distichlis spicata), and/or povertyweed and other invasive species—to expand or occupy interspaces.
Beardless wildrye can spread by rhizomatous rooting and is tolerant of grazing and increases under grazing pressure (USDA, 1988).
During settlement, many of the cattle in the Great Basin were wintered on extensive basin wildrye stands, and, due to basin wildrye’s sensitivity to spring use, many stands were decimated by the early 20th century (Young et al., 1975). Less palatable species such as greasewood, rabbitbrush, and saltgrass, and invasive, non-native/weedy species such as povertyweed, Russian thistle (Salsola kali), mustards, and cheatgrass increased in dominance (Roundy, 1985). Spring defoliation of basin wildrye and/or consistent, heavy grazing during the growing season have been found to significantly reduce basin wildrye production and density (Krall et al., 1971). Thus, inadequate rest and recovery from defoliation can cause a decrease in basin wildrye and an increase in rabbitbrush, greasewood, saltgrass, and non-native weeds (Young et al., 1975; Roundy, 1985). Additionally, natural Great Basin wildrye seed viability is low and seedlings lack vigor (Young & Evans, 1981). Roundy (1985) found that although basin wildrye is adapted to seasonally dry, saline soils, high and frequent spring precipitation is necessary to establish it from seed. This suggests that establishment of natural basin wildrye seedlings occurs only during years of unusually high precipitation. Therefore, reestablishment of a stand that has been decimated by grazing may be episodic.
Nevada bluegrass is an important forage source because of its early spring growth and palatability. It rates as excellent forage for cattle and horses, good to excellent for sheep, good for elk, and fair to good for deer. This grass, with the exception of Sandberg bluegrass, is the most drought-tolerant of the bluegrasses. Remarkably deep, extensive, and fibrous roots enable this plant to grow on rather dry sites and to endure extended droughts. Unlike the related Sandberg bluegrass, this plant succumbs to heavy grazing and trampling and has been reduced in extent on many western ranges due to over-utilization (USDA, 1988).
Basin big sagebrush/basin wildrye communities provide cover and food for large ungulates, upland game birds, and smaller wildlife. Because of its tall, heavy growth, basin wildrye provides forage for elk (Cervus canadensis) and other big game in the winter when snow cover is more than two feet (Plummer et al., 1968).
Wild ungulates use basin big sagebrush for cover and feed. Mule deer, pronghorn (Antilocarpra americana), and elk will browse basin big sagebrush from autumn through early spring (Wambolt et al., 1994). Early and mid-seral basin big sagebrush provide forage and protection from predators for mule deer (Wildlife Action Plan Team, 2012). Mule deer preference for the shrub varies seasonally. Basin big sagebrush was used more by mule deer populations in Oregon and Utah in winter than by the same populations in fall (Sheehy & Winward, 1981; Welch et al., 1981). This could be because basin big sagebrush is consumed as a last resort plant and browsed when plants considered more palatable were no longer available (Welch et al., 1981). Elk and pronghorn antelope will browse basin big sagebrush in areas where mountain and Wyoming big sagebrush are unavailable (Beale & Smith, 1970 Wambolt, 1996).
A study by Brown et al. (1977) determined that desert bighorn sheep preferred big sagebrush over other shrub types; however, the variety was not noted.
These plants communities provide cover and food for smaller desert wildlife such as lagomorphs and rodents. Pygmy rabbits (Brachylagus idahoensis) rely on tall basin big sagebrush for shelter and food throughout the year (Green & Flinders, 1980; White et al., 1982; Wildlife Action Plan Team, 2012). A study by Larrison and Johnson (1973) captured deer mice (Peromyscus maniculatus) in big sagebrush communities more than any other plant community, suggesting the mice prefer these plant communities for cover over other plant communities.
Basin big sagebrush serves as valuable habitat for native birds. Studies have suggested that sage grouse use basin big sagebrush for cover and food where mountain and Wyoming big sagebrush are absent (Welch et al., 1991). Birds such as Brewer’s sparrows (Spizella breweri) are considered dependent on sagebrush communities for cover and will nest in basin big sagebrush. Thus, when basin big sagebrush communities are converted to agriculture fields, Brewer’s sparrow populations can decline due to loss of habitat (Knick et al., 2003). In fact, mature basin big sagebrush act as nesting structures, protection from predators, and thermal cover for Greater sage grouse (Centrocercus urophasianus), loggerhead shrike (Lanius ludovicianus), sage sparrow (Artemisiospiza nevadensis), Brewer’s sparrow, and sage thrasher (Oreoscoptes montanus) (Wildlife Action Plan Team, 2012). The plant also acts as important cover for game birds such as the gray partridge (Perdix perdix), mountain quail (Oreotyx pictus), and mourning doves (Zenaida macroura), as well as passerines such as towhees (Pipilo spp.) and finches (Haemorhous spp.) that inhabit arid rangelands in the West (Dobbs et al., 2012; Booth, 1985).
Changes in plant community composition caused by human activity, invasive weeds, and fire frequency could affect the distribution and presence of wildlife species.
References:
Beckstead, J., and Augspurger, C. K. 2004. An experimental test of resistance to cheatgrass invasion: limiting resources at different life stages. Biological Invasions 6(4):417-432.
Belsky, A. J., Matzke, A., and S. Uselman. 1999. Survey of livestock influences on stream and riparian ecosystems in the western United States. Journal of Soil and Water Conservation 54:419-431.
Bentz, B., D. Alston, and T. Evans. 2008. Great Basin Insect Outbreaks. In: J. Chambers, N. Devoe, A. Evenden (eds.). Collaborative Management and Research in the Great Basin -- Examining the issues and developing a framework for action Gen. Tech. Rep. RMRS-GTR-204. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, CO. Pages 45-48.
Brown, K. W., D. D. Smith, and R. P. McQuivey. Food Habits of Desert Bighorn Sheep in Nevada, 1956- 1976. In: C. Douglas, R. Valdez, J. David Leslie, and T. O'Farrel (Eds.), Desert Bighorn Council 1977 Transactions. 1977, April 6-8. Las Cruces, New Mexico. Desert Bighorn Council, Pages 32-61.
Chambers, J. C., J. R. Miller, D. Germanoski, and D. A. Weixelman. 2004. Process-Based Approaches for Managing and Restoring Riparian Ecosystems. Pages 261-292. In Chambers, J. C. and Miller, J. R. (Eds.), Great Basin Riparian Ecosystems: Ecology, Management, and Restoration. Island Press, Washington D. C.
Comstock, J. P. and J. R. Ehleringer. 1992. Plant adaptation in the Great Basin and Colorado Plateau. Western North American Naturalist 52(3):195-215.
Dobbs, R. C., P. R. Martin, and T. E. Martin. 2020. Green-tailed Towhee (Pipilo chlorurus). In A. F. Poole (Ed.), The Birds of North America Online. Cornell Lab of Ornithology, Ithaca, NY. https://doi.org/10.2173/bow.gnttow.01
Dobrowolski, J. P., M. M. Caldwell, and J. H. Richards. 1990. Basin hydrology and plant root systems. Pages 243-292. In C. B. Osmond, L. F. Pitelka, and G. M. Hidy (Eds.), Plant biology of the basin and range. Springer-Verlag, New York.
Forman, R. T. T., and R. D. Deblinger. 2000. The Ecological Road-Effect Zone of a Massachusetts (U.S.A.) Surburban Highway. Conservation Biology 14(1):36-46.
Furniss, M. M. and W. F. Barr. 1975. Insects affecting important native shrubs of the northwestern United States Gen. Tech. Rep. INT-19. Intermountain Forest and Range Experiment Station, U.S. Department of Agriculture, Forest Service. Ogden, UT. 68 p.
Goodrich, S., E. D. McArthur, and A. H. Winward. 1985. A new combination and a new variety in Artemisia tridentata The Great Basin Naturalist 45(1):99-104.
Johnson, B. G., Johnson, D. W., Chambers, J. C., and B. R. Blank. 2011. Fire effects on the mobilization and uptake of nitrogen by cheatgrass (Bromus tectorum L.). Plant and Soil 341(1-2):437-445.
Larrison, E. J., and D. R. Johnson. 1973. Density changes and habitat affinities of rodents of shadscale and sagebrush associations. Great Basin Naturalist 33(4):255-264.
MacMahon, J. A. 1980. Ecosystems over time: succession and other types of change. In R. Warning (Ed.), Proceedings--Forests: fresh perspectives from ecosystem analyses. Biological Colloquium. Corvallis, OR: Oregon State University. Pages 27-58.
Majerus, M. E. 1992. High-stature grasses for winter grazing. Journal of Soil and Water Conservation 47(3):224-225.
Miller, R. F., J. C. Chambers, D. A. Pyke, F. B. Pierson, and C. J. Williams. 2013. A Review of Fire Effects on Vegetation and Soils in the Great Basin Region: Response and Ecological Site Characteristics. USDA Forest Service, Rocky Mountain Research Station Fort Collins, CO.
Ogle, D. G., D. Tilley, and L. S. John. 2012. Plant Guide for basin wildrye (Leymus cinereus). USDA-Natural Resources Conservation Service, Aberdeen Plant Materials Center, Aberdeen, ID.
Plummer, A. P., D. R. Christensen, and S. B. Monsen. 1968. Restoring Big-Game Range in Utah. Pub. No. 68-3. Utah Division of Fish and Game, Salt Lake City, UT. 183 p.
Reynolds, T. D., and L. Fraley. 1989. Root profiles of some native and exotic plant species in southeastern Idaho. Environmental and Experimental Botany 29(2):241-248.
Rosgen, D. L. 2006. Watershed Assessment of River Stability and Sediment Supply (WARSSS). Wildland Hydrology Books, Fort Collins, CO.
Roundy, B. A. 1985. Germination and Seedling Growth of Tall Wheatgrass and Basin Wildrye in Relation to Boron. Journal of Range Management 38(3):270-272.
Trombulak, S. C., and C. A. Frissell. 2000. Review of Ecological Effects of Roads on Terrestrial and Aquatic Communities. Conservation Biology 14(1):18-30.
[USDA] United States Department of Agriculture. 1988. Range Plant Handbook (Reproduction of the 1937 edition). Dover Publications, Inc.: New York. 848 pp.
[USDA-NRCS] U.S. Department of Agriculture, National Resource Conservation Service. 1998. Stream Visual Assessment Protocol. NWCC Technical Note 99-1.
Welch, B. L., F. J. Wagstaff, and J. A. Roberson. 1991. Preference of wintering sage grouse for big sagebrush. Journal of Range Management 44(5):462-465.
Whisenant, S. G. 1999. Repairing damaged wildlands: a process-oriented, landscape-scale approach (Vol. 1). Cambridge, UK: Cambridge University Press. 312 p.
Wildlife Action Plan Team. 2012. Nevada Wildlife Action Plan. Nevada Department of Wildlife, Reno, NV.
Young, J. A., R. A. Evans, and P. T. Tueller. 1975. Great Basin plant communities- pristine and grazed. Holocene environmental change in the Great Basin. Nevada Archeological Survey Research Paper 6. Pages 187-212.
Young, R. P. 1983. Fire as a vegetation management tool in rangelands of the Intermountain region. In Monsen, S.B. and N. Shaw (Eds), Managing Intermountain rangelands—improvement of range and wildlife habitats: Proceedings. 1981, September 15-17; Twin Falls, ID; 1982, June 22-24; Elko, NV. Gen. Tech. Rep. INT-157. Ogden, UT. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Pages 18-31.
Zschaechner, G. A. 1985. Studying rangeland fire effects: a case study in Nevada. In K. Sanders and J. Durham (Eds.), Rangeland Fire Effects: A Symposium. 1984, November 27-29. USDI-BLM Idaho State Office, Boise, ID. Pages 66-84.
Major Land Resource Area
MLRA 023X
Malheur High Plateau
Correlated Map Unit Components
21659146, 21660589, 21660480, 21660496, 21660509, 21660612, 21660497, 21660386, 21660628, 21660650, 21500299, 21500300, 21500317, 21500322, 21500301, 21500781, 21501623, 21501621, 21500437, 21501277, 21500782, 21501044, 21500789, 21501445, 21501448, 21500973, 21500499, 21500522, 21500496, 21500515, 21500495, 21500256, 21501171, 21500608, 21500528, 21500742, 21500792, 21481865, 21481867, 21590135, 21589477, 21590058, 21589523, 21589602, 21590052, 21590060, 21589499, 21589965, 21589491, 21589493, 21590591, 21590406, 21590409, 21590408, 21590532, 21605058, 21604273, 21604347, 21730195, 22168089, 22168373, 22170926, 22171242, 22171114, 22171188, 22171179, 22171035, 22170442, 22170734, 22170828, 22170825, 22170820, 22171230, 22170645, 22170641, 22170637, 22170632, 22171090, 22170633, 22170589, 22176834, 22176503, 22175206, 22176474, 22176477, 22176923, 22176196, 22175755, 22175611, 22176977, 22175779, 22176327, 22176653, 22176654, 22175954, 22176113, 22175843, 22176139, 22176140, 22176758, 22177674, 22177448
Stage
Provisional
Contributors
T Stringham (UNR under contract with BLM)
DMP
Click on box and path labels to scroll to the respective text.