Natural Resources
Conservation Service
Ecological site R026XY005NV
LOAMY 12-14 P.Z.
Last updated: 4/10/2024
Accessed: 02/27/2026
General information
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
MLRA notes
Major Land Resource Area (MLRA): 026X–Carson Basin and Mountains
The area lies within western Nevada and eastern California, with about 69 percent being within Nevada, and 31 percent being within California. Almost all this area is in the Great Basin Section of the Basin and Range Province of the Intermontane Plateaus. Isolated north-south trending mountain ranges are separated by aggraded desert plains. The mountains are uplifted fault blocks with steep side slopes. Most of the valleys are drained by three major rivers flowing east across this MLRA. A narrow strip along the western border of the area is in the Sierra Nevada Section of the Cascade-Sierra Mountains Province of the Pacific Mountain System. The Sierra Nevada Mountains are primarily a large fault block that has been uplifted with a dominant tilt to the west. This structure leaves an impressive wall of mountains directly west of this area. This helps create a rain shadow affect to MLRA 26. Parts of this eastern face, but mostly just the foothills, mark the western boundary of this area. Elevations range from about 3,806 feet (1,160 meters) on the west shore of Pyramid Lake to 11,653 feet (3,552 meters) on the summit of Mount Patterson in the Sweetwater Mountains.
Valley areas are dominantly composed of Quaternary alluvial deposits with Quaternary playa or alluvial flat deposits often occupying the lowest valley bottoms in the internally drained valleys, and river deposited alluvium being dominant in externally drained valleys. Hills and mountains are dominantly Tertiary andesitic flows, breccias, ash flow tuffs, rhyolite tuffs or granodioritic rocks. Quaternary basalt flows are present in lesser amounts, and Jurassic and Triassic limestone and shale, and Precambrian limestone and dolomite are also present in very limited amounts. Also of limited extent are glacial till deposits along the east flank of the Sierra Nevada Mountains, the result of alpine glaciation.
The average annual precipitation in this area is 5 to 36 inches (125 to 915 millimeters), increasing with elevation. Most of the rainfall occurs as high-intensity, convective storms in spring and autumn. Precipitation is mostly snow in winter. Summers are dry. The average annual temperature is 37 to 54 degrees F (3 to 12 degrees C). The freeze-free period averages 115 days and ranges from 40 to 195 days, decreasing in length with elevation.
The dominant soil orders in this MLRA are Aridisols and Mollisols. The soils in the area dominantly have a mesic soil temperature regime, an aridic or xeric soil moisture regime, and mixed or smectitic mineralogy. They generally are well drained, are clayey or loamy and commonly skeletal, and are very shallow to moderately deep.
This area supports shrub-grass vegetation characterized by big sagebrush. Low sagebrush and Lahontan sagebrush occur on some soils. Antelope bitterbrush, squirreltail, desert needlegrass, Thurber needlegrass, and Indian ricegrass are important associated plants. Green ephedra, Sandberg bluegrass, Anderson peachbrush, and several forb species also are common. Juniper-pinyon woodland is typical on mountain slopes. Jeffrey pine, lodgepole pine, white fir, and manzanita grow on the highest mountain slopes. Shadscale is the typical plant in the drier parts of the area. Sedges, rushes, and moisture-loving grasses grow on the wettest parts of the wet flood plains and terraces. Basin wildrye, alkali sacaton, saltgrass, buffaloberry, black greasewood, and rubber rabbitbrush grow on the drier sites that have a high concentration of salts.
Some of the major wildlife species in this area are mule deer, coyote, beaver, muskrat, jackrabbit, cottontail, raptors, pheasant, chukar, blue grouse, mountain quail, and mourning dove. The species of fish in the area include trout and catfish. The Lahontan cutthroat trout in the Truckee River is a threatened and endangered species.
LRU notes
The Sierra Influenced Ranges LRU is characterized by wooded great basin mountains with climatic and biotic affinities to the Sierra Nevada mountain range. The Sierra Influences Ranges LRU receives greater precipitation that the mountain ranges of central NV. Amount of precipitation varies in relation to the local strength of the Sierra NV rain shadow, characterized by pinyon and juniper trees. The White, Sweetwater, Pine Nut, Wassuk, and Virginia ranges of Nevada support varying amounts of Sierra Nevada flora, such as ponderosa pine. Elevations range from 1610 to 2420 meters and slopes range from 5 to 49 percent, with a median value of 22 percent. Frost free days (FFD) ranges from 92 to 163.
Ecological site concept
The Loamy 12-14” ecological site is the modal site that represents this DRG, as it has the most acres mapped. This site occurs on mountain sideslopes and toeslopes, and mountain valley fans on all aspects. Slopes range from 15 to 75 percent, but slope gradients of 30 to 50 percent are most typical. Elevations are 5,410 to 7,800 feet. Soils are typically deep to very deep and are well drained. Some soils are modified with high volumes of rock fragments throughout the soil profile. The shrub component of the plant community is dominated by mountain big sagebrush (Artemisia tridentata spp. vaseyana) and the herbaceous component is co-dominated by western needlegrass (Achnatherum occidentale ssp. occidentale), and Letterman’s needlegrass (Achnatherum lettermanii). Antelope bitterbrush (Purshia tridentata) is also a component of the shrub layer. Normal year production is 1500 lbs/ac.
Associated sites
| R026XY007NV |
STEEP NORTH SLOPE 14+ P.Z. |
|---|---|
| R026XY010NV |
LOAMY 10-12 P.Z. |
| R026XY023NV |
CLAYPAN 10-12 P.Z. |
| R026XY030NV |
LOAMY BOTTOM 8-12 P.Z. |
| R026XY039NV |
CLAYPAN 14+ P.Z. |
| R026XY053NV |
LOAMY 16+ P.Z. |
Similar sites
| R026XY018NV |
GRANITIC SOUTH SLOPE 10-12 P.Z. ACTH7-ACOCO codominant grasses |
|---|---|
| R026XY082NV |
MOUNTAIN LOAM 16+ P.Z. ACPI2-KOMA codominant grasses with ACLE9 |
| R026XY100NV |
STONY SLOPE 10-12 P.Z. Less productive site; ACTH7-POFE codominant |
| R026XY053NV |
LOAMY 16+ P.Z. More productive site; BRMA4 codominant grass |
| R026XY076NV |
MOUNTAIN SHOULDERS 16+ P.Z. ACLE9 dominant grass; less productive site |
| R026XY008NV |
GRANITIC FAN 10-12 P.Z. HECO26-ACHY codominant grasses; less productive site |
| R026XY089NV |
SOUTH SLOPE 12-14 P.Z. Less productive site |
| R026XY079NV |
GRANITIC SOUTH SLOPE 14+ P.Z. Less productive site |
| R026XY010NV |
LOAMY 10-12 P.Z. ACTH7 dominant grass; less productive site |
| R026XY006NV |
GRANITIC LOAM 14+ P.Z. Less productive site; soils derived from granitic parent material |
| R026XY038NV |
LOAMY SLOPE 14+ P.Z. Less productive site; PUTR2 minor species |
| R026XY052NV |
SHALLOW LOAM 16+ P.Z. LEKI2 dominant grass |
| R026XY040NV |
GRAVELLY LOAM 14+ P.Z. PUTR2 dominant shrub |
| R026XY084NV |
DEEP LOAMY 14+ P.Z. ACOCO-PONE3 codominant; less productive site |
| R026XY026NV |
GRANITIC SLOPE 10-12 P.Z. ACTH7-ACSP12 codominant grasses |
| R026XY046NV |
GRANITIC SLOPE 12-14 P.Z. Less productive site |
| R026XY048NV |
LOAMY SLOPE 12-14 P.Z. ACTH7-ACOCO codominant; less productive site |
Table 1. Dominant plant species
| Tree |
Not specified |
|---|---|
| Shrub |
(1) Artemisia tridentata ssp. vaseyana |
| Herbaceous |
(1) Achnatherum occidentale ssp. occidentale |
Physiographic features
This site occurs on mountain sideslopes and toeslopes and mountain valley fans on all aspects. This site is restricted to concave positions on northerly aspects at lower elevations. Slopes range from 15 to 75 percent, but slope gradients of 30 to 50 percent are most typical. Elevations are 5400 to 7800 feet.
Table 2. Representative physiographic features
| Landforms |
(1)
Mountain slope
(2) Mountain |
|---|---|
| Elevation | 5,410 – 7,800 ft |
| Slope | 15 – 75% |
| Aspect | Aspect is not a significant factor |
Climatic features
The climate associated with this site is subhumid with cool, dry summers and cold, wet winters. Average annual precipitation is 12 to over 14 inches. Mean annual air temperature is 40 to 45 degrees F. The average growing season is about 50 to 100 days.
Nevada’s climate is predominantly arid, with large daily ranges of temperature, infrequent severe storms, heavy snowfall in the higher mountains, and great location variations with elevation. Three basic geographical factors largely influence Nevada’s climate: continentality, latitude, and elevation. Continentality is the most important factor. The strong continental effect is expressed in the form of both dryness and large temperature variations. Nevada lies on the eastern, lee side of the Sierra Nevada Range, a massive mountain barrier that markedly influences the climate of the State. The prevailing winds are from the west, and as the warm moist air from the Pacific Ocean ascend the western slopes of the Sierra Range, the air cools, condensation occurs and most of the moisture falls as precipitation. As the air descends the eastern slope, it is warmed by compression, and very little precipitation occurs. The effects of this mountain barrier are felt not only in the West but throughout the state, with the result that the lowlands of Nevada are largely desert or steppes. The temperature regime is also affected by the blocking of the inland-moving maritime air. Nevada sheltered from maritime winds, has a continental climate with well-developed seasons and the terrain responds quickly to changes in solar heating.
Nevada lies within the mid-latitude belt of prevailing westerly winds which occur most of the year. These winds bring frequent changes in weather during the late fall, winter and spring months, when most of the precipitation occurs. To the south of the mid-latitude westerlies, lies a zone of high pressure in subtropical latitudes, with a center over the Pacific Ocean. In the summer, this high-pressure belt shifts northward over the latitudes of Nevada, blocking storms from the ocean. The resulting weather is mostly clear and dry during the summer and early fall, with scattered thundershowers. The eastern portion of the state receives significant summer thunderstorms generated from monsoonal moisture pushed up from the Gulf of California, known as the North American monsoon. The monsoon system peaks in August and by October the monsoon high over the Western U.S. begins to weaken and the precipitation retreats southward towards the tropics (NOAA 2004).
Table 3. Representative climatic features
| Frost-free period (characteristic range) | |
|---|---|
| Freeze-free period (characteristic range) | |
| Precipitation total (characteristic range) | 12-14 in |
| Frost-free period (average) | 75 days |
| Freeze-free period (average) | |
| Precipitation total (average) | 13 in |
Figure 1. Monthly average minimum and maximum temperature
Figure 2. Annual precipitation pattern
Figure 3. Annual average temperature pattern
Influencing water features
There are no influencing water features associated with this site.
Soil features
The soils associated with this site are typically moderately deep to very deep and well drained. The soils are formed in colluvium and or residuum derived from volcanic rock. The soils typically have a mollic epipedon and an argillic horizon. Available water capacity is medium to high. The soils are moist from fall through early summer when plant growth depletes soil moisture. The soil moisture regime is aridic bordering on xeric. The soil temperature regime is mesic and frigid. Some soils are modified with high volumes of rock fragments through the soil profile. Runoff is medium to very high. Soil series associated with this site include: Booford, Burnborough, Drit, Epvip, Glean, Hartig, Ister, Nire, Nosrac, Softscrabble, and Uhaldi.
A representative soil series is Nosrac, a loamy-skeletal, mixed, superactive, mesic Aridic Argixerolls. A mollic epipedon occurs from the soil surface to 46 cm and an argillic horizon occurs from 23 to 152 cm.
Table 4. Representative soil features
| Parent material |
(1)
Colluvium
–
volcanic rock
(2) Residuum – volcanic rock |
|---|---|
| Surface texture |
(1) Very stony loam (2) Very stony sandy loam |
| Family particle size |
(1) Loamy |
| Drainage class | Well drained |
| Permeability class | Moderately slow to moderately rapid |
| Soil depth | 20 – 84 in |
| Surface fragment cover <=3" | 15 – 30% |
| Surface fragment cover >3" | 3 – 40% |
| Available water capacity (0-40in) |
2.4 – 4.9 in |
| Calcium carbonate equivalent (0-40in) |
Not specified |
| Electrical conductivity (0-40in) |
2 mmhos/cm |
| Sodium adsorption ratio (0-40in) |
Not specified |
| Soil reaction (1:1 water) (0-40in) |
5.6 – 7.3 |
| Subsurface fragment volume <=3" (Depth not specified) |
15 – 31% |
| Subsurface fragment volume >3" (Depth not specified) |
3 – 29% |
Ecological dynamics
The ecological sites in this DRG are dominated by deep-rooted cool season perennial bunchgrasses and long-lived shrubs (50+ years) with high root to shoot ratios. The dominant shrubs usually root to the full depth of the winter-spring soil moisture recharge, which ranges from 1.0 to over 3.0 m. (Comstock and Ehleringer 1992). Root length of mature sagebrush plants was measured to a depth of two meters in alluvial soils in Utah (Richards and Caldwell 1987). Tap roots of antelope bitterbrush have been documented from 4.5 to 5.4 m in length (McConnell 1961). These shrubs have a flexible generalized root system with development of both deep taproots and laterals near the surface (Comstock and Ehleringer 1992).
The Great Basin sagebrush communities have high spatial and temporal variability in precipitation both among years and within growing seasons. Nutrient availability is typically low but increases with elevation and closely follows moisture availability. The moisture resource supporting the greatest amount of plant growth is usually the water stored in the soil profile during the winter. The invasibility of plant communities is often linked to resource availability. Disturbance can decrease resource uptake due to damage or mortality of the native species and depressed competition, or it can increase resource uptake by the decomposition of dead plant material following disturbance. The invasion of sagebrush communities by cheatgrass (Bromus tectorum) has been linked to disturbances (fire, abusive grazing) that have resulted in fluctuations in resources (Chambers et al 2007). Dobrowolski et al. (1990) cite multiple authors on the extent of the soil profile exploited by the competitive exotic annual cheatgrass. Specifically, the depth of rooting is dependent on the size the plant achieves, and in competitive environments cheatgrass roots were found to penetrate only 15 cm, whereas isolated plants and pure stands were found to root at least 1 m in depth with some plants rooting as deep as 1.5 to 1.7 m.
Periodic drought regularly influences sagebrush ecosystems and drought duration and severity has increased throughout the 20th century in much of the Intermountain West. Major shifts away from historical precipitation patterns have the greatest potential to alter ecosystem function and productivity. Species composition and productivity can be altered by the timing of precipitation and water availability within the soil profile (Bates et al. 2006).
Native insect outbreaks are also important drivers of ecosystem dynamics in big sagebrush communities. Climate influences the timing of insect outbreaks, especially a sagebrush defoliator, Aroga moth (Aroga websteri). Aroga moth infestations have occurred in the Great Basin in the 1960s, early 1970s, and have been ongoing in Nevada since 2004 (Bentz et al. 2008). Thousands of acres of big sagebrush have been impacted, with partial to complete die-off observed. Aroga moth can partially or entirely kill individual plants or entire stands of big sagebrush (Furniss and Barr 1975).
Mountain big sagebrush and antelope bitterbrush are generally long-lived; therefore, it is not necessary for new individuals to recruit every year for perpetuation of the stand. Infrequent large recruitment events and simultaneous low, continuous recruitment is the foundation of population maintenance (Noy-Meir 1973). Survival of the seedlings is dependent on adequate moisture conditions.
The perennial bunchgrasses that are co-dominant with the shrubs in this group include western needlegrass, Letterman’s needlegrass, and Columbia needlegrass. Basin wildrye is an important species as well. These species generally have somewhat shallower root systems than the shrubs, but root densities are often as high as or higher than those of shrubs in the upper 0.5 m and taper off more rapidly than shrubs. General differences in root depth distributions between grasses and shrubs result in resource partitioning in these shrub/grass systems.
Letterman needlegrass is an erect, densely tufted perennial bunchgrass that forms large clumps. It is found on dry soils in a variety of vegetation communities, including, high elevation meadows, subalpine grasslands, open areas underneath aspen, and in sagebrush communities. It grows best on loamy soils that are greater than 20 cm deep (Dittberner and Olson 1983).
Basin wildrye is a large, cool-season perennial bunchgrass with an extensive deep coarse fibrous root system (Reynolds and Fraley 1989). Clumps may reach up to six feet in height (Ogle et al. 2012b). Basin wildrye does not tolerate long periods of inundation; it prefers cycles of wet winters and dry summers and is most commonly found in deep soils with high water holding capacities or seasonally high water tables (Ogle et al. 2012b, Perryman and Skinner 2007).
Infilling by singleleaf pinyon (Pinus monophylla) and Utah juniper (Juniperus osteosperma) may also occur with an extended fire return interval. Eventually, singleleaf pinyon and Utah juniper will dominate the site and mountain big sagebrush will be severely reduced along with the herbaceous understory. Bluegrasses (Poa spp.) and bottlebrush squirreltail (Elymus elymoides) may remain underneath trees on north-facing slopes. The potential for soil erosion increases as the pinyon and/or juniper woodland matures and the understory plant community cover declines.
Millions of acres in the arid and semi-arid West were brush-beaten and planted with crested wheatgrass in the mid 1900’s for the purpose of competing with weed species and increasing grass production on rangelands. Success and longevity of these seeding projects have been mixed (Williams et al. 2017). Crested wheatgrass is a cool-season, medium height, exotic perennial bunchgrass native to Asia. Sites within this DRG may exhibit an understory of crested wheatgrass in areas where historical seedings have occurred or where crested wheatgrass has been used in post-fire rehabilitation seedings.
The ecological sites in this DRG have low to moderate resilience to disturbance and resistance to invasion. Resilience increases with elevation, aspect, increased precipitation and increased nutrient availability. Long-term disturbance response may be influenced by small differences in landscape topography. North slopes are also more resilient than south slopes because lower soil surface temperatures operate to keep moisture content higher on northern exposures. Six possible alternative stable states have been identified for this DRG.
Invasive Annual Grasses:
The species most likely to invade these sites is cheatgrass. Cheatgrass is a cool season annual grass that maintains an advantage over native plants in part because it is a prolific seed producer, can germinate in the autumn or spring, tolerates grazing, and increases with frequent fire (Klemmedson and Smith 1964, Miller et al. 1999). Cheatgrass originated from Eurasia and was first reported in North America in the late 1800s (Mack and Pyke 1983; Furbush 1953). Pellant and Hall (1994) found 3.3 million acres of public lands dominated by cheatgrass and suggested that another 76 million acres were susceptible to invasion by winter annuals including cheatgrass and medusahead.
Recent modeling and empirical work by Bradford and Lauenroth (2006) suggests that seasonal patterns of precipitation input and temperature are also key factors determining regional variation in the growth, seed production, and spread of invasive annual grasses. The phenomenon of cheatgrass “die-off” provides opportunities for restoration of perennial and native species (Baughman et al. 2016, Baughman et al. 2017). The causes of these events are not fully understood, but there is ongoing work to try to predict where they occur, in the hopes of aiding conservation planning (Weisberg et al. 2017, Brehm 2019).
Methods to control cheatgrass include herbicide, fire, targeted grazing, and seeding. Mapping potential or current invasion vectors is a management method designed to increase the cost effectiveness of control methods. Spraying with herbicide (Imazapic or Imazapic + glyphosate) and seeding with crested wheatgrass and Sandberg bluegrass has been found to be more successful at combating cheatgrass (and medusahead) than spraying alone (Sheley et al. 2012). To date, most seeding success has occurred with non-native wheatgrass species. Perennial grasses, especially crested wheatgrass, are able to suppress cheatgrass growth when mature (Blank et al. 2020). Where native bunchgrasses are missing from the site, revegetation of annual grass invaded rangelands has been shown to have a higher likelihood of success when using introduced perennial bunchgrasses such as crested wheatgrass (Clements et al. 2017, Davies et al. 2015). Butler et al. (2011) tested four herbicides (Imazapic, Imazapic + glyphosate, rimsulfuron, and sulfometuron + Chlorsulfuron) for suppression of cheatgrass, medusahead and ventenata (North Africa grass, Ventenata dubia) within residual stands of native bunchgrass. Additionally, they tested the same four herbicides followed by seeding of six bunchgrasses (native and non-native) with varying success (Butler et al. 2011). Herbicide-only treatments appeared to remove competition for established bluebunch wheatgrass by providing 100% control of ventenata and medusahead and greater than 95% control of cheatgrass (Butler et al. 2011). Caution in using these results is advised, as only one year of data was reported.
In considering the combination of pre-emergent herbicide and prescribed fire for invasive annual grass control, it is important to assess the tolerance of desirable brush species to the herbicide being applied. Vollmer and Vollmer (2008) tested the tolerance of mountain mahogany (Cercocarpus montanus), antelope bitterbrush, and multiple sagebrush species to three rates of Imazapic with and without methylated seed oil as a surfactant. They found a cheatgrass control program in an antelope bitterbrush community should not exceed Imazapic at 8 oz./ac with or without surfactant. Sagebrush, regardless of species or rate of application, was not affected. However, many environmental variables were not reported in this study and managers should install test plots before broad scale herbicide application is initiated.
Fire Ecology:
Fire is believed to be the dominant disturbance force in natural big sagebrush communities. Several authors suggest pre-settlement fire return intervals in mountain big sagebrush communities varied from 15 to 25 years (Burkhardt and Tisdale 1969, Houston 1973, and Miller et al. 2000). Kitchen and McArthur (2007) suggest a mean fire return interval of 40 to 80 years for mountain big sagebrush communities. Natural fire return intervals are estimated to vary between less than 35 years up to 100 years in sagebrush ecosystems with basin wildrye (Brown and Smith 2000). The range from 15 to 80 years is probably more accurate and reflects the differences in elevation and precipitation where mountain big sagebrush communities occur. On a landscape scale, multiple seral stages were represented in a mosaic reflecting periodic reoccurrence of fire and other disturbances (Crawford et al. 2004). Post-fire hydrologic recovery and resilience is primarily influenced by pre-fire site conditions, fire severity, and post-fire weather and land use that relate to vegetation recovery. Fire adaptation by herbaceous species is generally superior to the dominant shrubs, which are typically killed by fire. Sites with low abundances of native perennial grasses and forbs typically have reduced resiliency following disturbance and are less resistant to invasion or increases in cheatgrass (Miller et al. 2013).
Mountain big sagebrush is killed by fire (Neunschwander 1980, Blaisdell et al. 1982) and does not resprout (Blaisdell 1953). Post-fire regeneration occurs from seed and will vary depending on site characteristics, seed source, and fire characteristics. Mountain big sagebrush seedlings can grow rapidly and may reach reproductive maturity within 3 to 5 years (Bunting et al. 1987). Mountain big sagebrush may return to pre-burn density and cover within 15 to 20 years following fire, but establishment after severe fires may proceed more slowly (Bunting et al. 1987).
Antelope bitterbrush is moderately fire tolerant (McConnell and Smith 1977). It regenerates by seed and resprouting (Blaisdell and Mueggler 1956, McArthur et al. 1982), however, sprouting ability is highly variable and has been attributed to genetics, plant age, phenology, soil moisture and texture, and fire severity (Blaisdell and Mueggler 1956, Blaisdell et al. 1982, Clark et al. 1982, Cook et al. 1994). Bitterbrush sprouts from a region on the stem approximately 1.5 inches above and below the soil surface; the plant rarely sprouts if the root crown is killed by fire (Blaisdell and Mueggler 1956). Low intensity fires may allow for bitterbrush to sprout; however, community response also depends on soil moisture levels at time of fire (Murray 1983). Lower soil moisture allows more charring of the stem below ground level (Blaisdell and Mueggler 1956), thus sprouting will usually be more successful after a spring fire than after a fire in summer or fall (Murray 1983, Busse et al. 2000, Kerns et al. 2006). If cheatgrass is present, bitterbrush seedling success is much lower. The factor that most limits establishment of bitterbrush seedlings is competition for water resources with the invasive species cheatgrass (Clements and Young 2002).
The effect of fire on bunchgrasses relates to culm density, culm-leaf morphology, and the size of the plant. The initial condition of bunchgrasses within the site along with seasonality and intensity of the fire all factor into the individual species response. For most forbs and grasses the growing points are located at or below the soil surface providing relative protection from disturbances which decrease above ground biomass, such as grazing or fire. Thus, fire mortality is more correlated to duration and intensity of heat which is related to culm density, culm-leaf morphology, size of plant and abundance of old growth (Young 1983, Wright 1971). Plant response will vary depending on post-fire soil moisture availability.
Columbia needlegrass resprouts quickly after fire (Monsen et al. 2004). Fall burning has been shown to increase seed production in Columbia needlegrass (Patton et al. 1988). Letterman’s needlegrass recovers well after fire (Monsen et al. 2004). Emergence of western needlegrass seeds was shown to significantly improve with additions of smoke and burned soil (Blank and Young 1996).
Basin wildrye is relatively resistant to fire, particularly dormant season fire, as plants sprout from surviving root crowns and rhizomes (Zschaechner 1985). Miller et al. (2013) reported increased total shoot and reproductive shoot densities in the first year following fire, although by year two there was little difference between burned and control treatments.
Bottlebrush squirreltail is considered to be relatively tolerant to fire due to its small size, coarse stems, and sparse leafy material (Britton et al. 1990). Post-fire regeneration occurs from surviving root crowns and from on- and off-site seed sources. Bottlebrush squirreltail has the ability to produce large numbers of seeds with high germination rates when exposed to the correct environmental cues (Young and Evans 1977). Early spring growth and ability to grow at low temperatures contribute to the persistence of bottlebrush squirreltail on sites with high cover of cheatgrass (Hironaka and Tisdale 1973).
Cheatgrass accumulates fuel loads that foster frequent fires (Davies and Svejcar 2008). Invasion by annual grasses can alter the fire cycle by increasing fire size, rate of spread, numbers of individual fires, fire season length, and likelihood of fires spreading into native or managed ecosystems (D’Antonio and Vitousek 1992, Brooks et al. 2004). Areas dominated with cheatgrass are estimated to have a fire return interval of 3 to 5 years (Whisenant 1990). The mechanisms by which invasive annual grasses alter fire regimes likely interact with climate. For example, cheatgrass cover and biomass vary with climate (Chambers et al. 2007) and are promoted by wet and warm conditions during the fall and spring. Invasive annual species have been shown able to take advantage of high N availability following fire through higher growth rates and increased seedling establishment relative to native perennial grasses (Monaco et al. 2003).
Catastrophic wildfire in Utah juniper and/or singleleaf pinyon dominated sites may lead to an annual weed dominated site. Depending on fire severity, rabbitbrush and snowberry may increase after fire. Rubber rabbitbrush (Ericameria nauseosa) is top-killed by fire, but can resprout after fire and can also establish from seed (Young 1983). Douglas’ rabbitbrush (Chrysothamnus viscidiflorus) is top-killed by fire, but sprouts vigorously after fire (Kuntz 1982, Akinsoji 1988). Snowberry is also top-killed by fire, but resprouts after fire from rhizomes (Noste and Bushey 1987). If arrowleaf balsamroot (Balsamorhiza sagittata) or mule-ears (Wyethia amplexicaulis) is common before fire, they will increase after fire. These species may increase with heavy grazing as well.
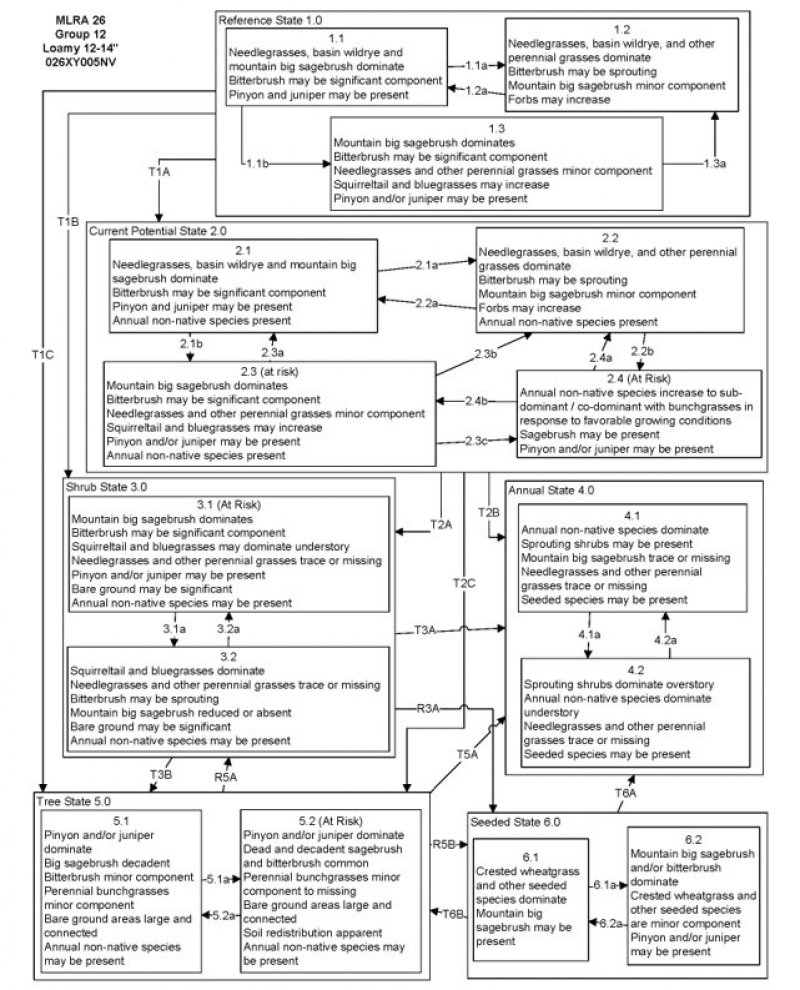
State and Transition Model Narrative Group 12:
This is a text description of the states, phases, transitions, and community pathways possible in the State and Transition model for the MLRA 26 Disturbance Response Group 12. Site included in this DRG are: R026XY005NV, R026XY105NV, R026XY046NV, R026XY006NV, R026XY048NV, R026XY111NV, R026XY040NV, R026XY106NV, R026XY089NV, R026XF057CA, R026XF063CA, and R026XF064CA.
Reference State 1.0:
The Reference State 1.0 represents the natural range of variability of this site under pristine conditions. The reference state has three general community phases: a shrub-grass dominant phase, a perennial grass dominant phase and a shrub dominant phase. State dynamics are maintained by interactions between climatic patterns and disturbance regimes. Negative feedbacks enhance ecosystem resilience and contribute to the stability of the state. These include the presence of all structural and functional groups, low fine fuel loads, and retention of organic matter and nutrients. Plant community phase changes are primarily driven by fire, periodic drought, and/or insect or disease attack.
Community Phase 1.1:
Needlegrasses, basin wildrye, and mountain big sagebrush dominate the site. Bitterbrush may be a significant component. Pinyon and juniper may be present.
Community Phase Pathway 1.1a, from phase 1.1 to 1.2:
Fire would decrease or eliminate the overstory of sagebrush and allow for the perennial bunchgrasses and perennial forbs to dominate the site. Fires would typically be small and patchy due to low fuel loads. Low severity fire creates sagebrush/grass mosaic. High severity fire significantly reduces sagebrush cover and leads to early/mid-seral community, dominated by grasses and forbs.
Community Phase Pathway 1.1b, from phase 1.1 to 1.3:
Time and lack of disturbance such as fire or drought allow for an increase in mountain big sagebrush. Excessive herbivory and/or long-term drought may also reduce perennial understory.
Community Phase 1.2:
This community phase is characteristic of a post-disturbance, early- to mid-seral plant community. Needlegrasses, basin wildrye, and perennial grasses and forbs dominate. Mountain big sagebrush is a minor component. Bitterbrush may be sprouting. Forbs may increase.
Community Phase Pathway 1.2a, from phase 1.2 to 1.1:
Time and lack of disturbance allows for sagebrush to reestablish.
Community Phase 1.3:
Mountain big sagebrush increases in the absence of disturbance and becomes dominant. Bitterbrush may be a significant component. Needlegrasses and other perennial grasses are reduced. Squirreltail and/or bluegrasses may increase. Pinyon and juniper may be present.
Community Phase Pathway 1.3a, from phase 1.3 to 1.2:
Fire. A low severity fire creates a sagebrush/grass mosaic, while a high-severity fire reduces sagebrush to trace amounts.
T1A: Transition from Reference State 1.0 to Current Potential State 2.0
Trigger: This transition is caused by the introduction of non-native annual weeds, such as cheatgrass, mustard and Russian thistle (Salsola tragus).
Slow variables: Over time the annual non-native plants will increase within the community, decreasing organic matter inputs from deep-rooted perennial bunchgrasses. This results in reductions in soil water availability for perennial bunchgrasses.
Threshold: Any amount of introduced non-native species causes an immediate decrease in the resilience of the site. Annual non-native species cannot be easily removed from the system and have the potential to significantly alter disturbance regimes from their historic range of variation.
T1B: Transition from Reference State 1.0 to Shrub State 3.0
Trigger: Inappropriate, long-term grazing of perennial bunchgrasses during the growing season would favor shrubs and initiate transition to Community Phase 3.1.
Slow variables: Long term decrease in deep-rooted perennial grass density.
Threshold: Loss of deep-rooted perennial bunchgrasses changes spatial and temporal nutrient cycling and nutrient redistribution and reduces soil organic matter and soil moisture.
T1C: Transition from Reference State 1.0 to Tree State 5.0
Trigger: Time and lack of disturbance or management action allows pinyon or juniper to dominate. This may be coupled with grazing management that favors tree establishment by reducing understory herbaceous competition for site resources.
Feedbacks and ecological processes: Trees increasingly dominate use of soil water, contributing to reductions in soil water availability to grasses and shrubs. Overtime, grasses and shrubs are outcompeted. Reduced herbaceous and shrub production slows soil organic matter inputs and increases soil erodibility through loss of cover and root structure.
Slow variables: Over time the abundance and size of trees will increase.
Threshold: Trees dominate ecological processes and the number of shrub skeletons exceed number of live shrubs.
Current Potential State 2.0:
This state is similar to the Reference State 1.0. Ecological function has not changed, however, the resiliency of the state has been reduced by the presence of invasive weeds. This state has four general community phases. Negative feedbacks enhance ecosystem resilience and contribute to the stability of the state. These include the presence of all structural and functional groups, low fine fuel loads and retention of organic matter and nutrients. Positive feedbacks decrease ecosystem resilience and stability of the state. These include the non-natives high seed output, persistent seed bank, rapid growth rate, ability to cross pollinate and adaptations for seed dispersal. Additionally, the presence of highly flammable, non-native species reduces State resilience because these species can promote fire where historically fire has been infrequent leading to positive feedbacks that further the degradation of the system.
Community Phase 2.1:
Needlegrasses, basin wildrye and mountain big sagebrush dominate the site. Bitterbrush may be a significant component. Pinyon and juniper may be present. Annual non-native species present.
Community Phase Pathway 2.1a, from phase 2.1 to 2.2:
Fire. Low severity fire creates sagebrush/grass mosaic while a high severity fire significantly reduces sagebrush cover and leads to early/mid-seral community dominated by grasses and forbs. Non-native annual species present.
Community Phase Pathway 2.1b, from phase 2.1 to 2.3:
Time, lack of disturbance, long-term drought, grazing management that favors shrubs or combinations of these would allow the sagebrush overstory to increase and dominate the site, causing a reduction in the perennial bunchgrasses.
Community Phase 2.2:
This community phase is characteristic of a post-disturbance, early-seral plant community. Needlegrasses and other perennial grasses and perennial forbs dominate. Mountain big sagebrush is a minor component. Bitterbrush may be sprouting. Forbs may increase. Annual non-native species are present.
Community Phase Pathway 2.2a, from phase 2.2 to 2.1:
Absence of disturbance over time allows for the sagebrush to recover. This may be combined with grazing management that favors shrubs.
Community Phase Pathway 2.2b, from Phase 2.2 to 2.4:
Higher than normal spring precipitation favors annual non-native species such as cheatgrass. Non-native annual species will increase in production and density throughout the site. Shrub removal or prescribed burning may increase invasive annuals. Perennial bunchgrasses may also increase in production.
Community Phase 2.3:
Mountain big sagebrush is dominant and the perennial understory is reduced. Bitterbrush may be a significant component. Bluegrass may increase. Pinyon and juniper may be present. Annual non-native species are present.
Community Phase Pathway 2.3a, from phase 2.3 to 2.1:
Low severity fire creates sagebrush/grass mosaic. Other disturbances/practices include brush management with minimal soil disturbance leading to a reduction in sagebrush.
Community Phase Pathway 2.3b, from phase 2.3 to 2.2:
High severity fire or brush management with minimal soil disturbance significantly reduces sagebrush and leads to early/mid-seral community.
Community Phase Pathway 2.3c, from phase 2.3 to 2.4:
Fall and spring growing season conditions that favor the germination and production of non-native annual grasses.
Community Phase 2.4:
This community is at risk of crossing to an annual state. Native bunchgrasses and forbs still comprise 50% or more of the understory annual production, however non-native annual grasses are nearly codominant. If this site originated from phase 2.3 there may be significant shrub cover as well. Annual production and abundance of these annuals may increase drastically in years with heavy spring precipitation. This site is susceptible to further degradation from grazing, drought, and fire.
Community Phase Pathway 2.4a, from phase 2.4 to 2.2:
Growing season conditions favoring perennial bunchgrass production and reduced cheatgrass production.
Community Phase Pathway 2.4b, from phase 2.4 to 2.3:
Growing season conditions favoring perennial bunchgrass production and reduced cheatgrass production.
T2A: Transition from Current Potential State 2.0 to Shrub State 3.0:
Trigger: Inappropriate, long-term grazing of perennial bunchgrasses during growing season would favor shrubs and initiate transition to Community Phase 3.1.
Slow variables: Long term declines in deep-rooted perennial grass density.
Threshold: Loss of deep-rooted perennial bunchgrasses changes spatial and temporal nutrient cycling and nutrient redistribution and reduces soil organic matter.
T2B: Transition from Current Potential State 2.0 to Annual State 4.0:
Trigger: Fire and/or multiple fires lead to plant community phase 4.1, inappropriate grazing management that favors shrubs in the presence of non-native annual species leads to community phase 4.2.
Slow variables: Increased production and cover of non-native annual species.
Threshold: Loss of deep-rooted perennial bunchgrasses and shrubs truncates, spatially and temporally, nutrient capture and cycling within the community. Increased, continuous fine fuels from annual non-native plants modify the fire regime by changing intensity, size and spatial variability of fires.
T2C: Transition from Current Potential 2.0 to Tree State 5.0:
Trigger: Time and lack of disturbance or management action allows pinyon and juniper to dominate. This may be coupled with grazing management that favors tree establishment by reducing understory herbaceous competition for site resources.
Feedbacks and ecological processes: Trees increasingly dominate use of soil water, contributing to reductions in soil water availability to grasses and shrubs. Overtime, grasses and shrubs are outcompeted. Reduced herbaceous and shrub production slows soil organic matter inputs and increases soil erodibility through loss of cover and root structure.
Slow variables: Over time the abundance and size of trees will increase.
Threshold: Trees dominate ecological processes and number of shrub skeletons exceed the number of live shrubs.
Shrub State 3.0:
This state has two community phases: a mountain big sagebrush dominated phase and a squirreltail-dominated phase. This state is a product of many years of heavy grazing during time periods harmful to perennial bunchgrasses. Squirreltail will increase with a reduction in deep rooted perennial bunchgrass competition and become the dominant grass. Sagebrush and bitterbrush dominate the overstory. Sagebrush canopy cover is high and sagebrush may be decadent, reflecting stand maturity and lack of seedling establishment due to competition with mature plants. The shrub overstory and bluegrass understory dominate site resources such that soil water, nutrient capture, nutrient cycling and soil organic matter are temporally and spatially redistributed.
Community Phase 3.1:
This site is at risk of transitioning to another state. Mountain big sagebrush, possibly decadent, dominates the overstory. Antelope bitterbrush may be a significant component. Deep-rooted perennial bunchgrasses are present in only trace amounts and may be absent from the community. Squirreltail may be dominant in the understory. Understory may be sparse, with bare ground increasing. Pinyon and/or juniper may be present as a result of encroachment from neighboring sites and lack of disturbance. Annual non-native species are present to increasing.
Community Phase Pathway 3.1a, from phase 3.1 to 3.2:
Fire reduces or eliminates the overstory of sagebrush. Non-native annual species increase with higher-than-normal spring precipitation.
Community Phase 3.2:
Squirreltail dominates the site. Needlegrasses and other perennial grasses are reduced. Mountain big sagebrush is reduced or missing. Bitterbrush may be sprouting. Annual non-native species are increasing and may be co-dominant in the understory.
Community Phase Pathway 3.2a, from phase 3.2 to 3.1:
Absence of disturbance over time would allow for sagebrush and other shrubs to recover.
T3A: Transition from Shrub State 3.0 to Annual State 4.0:
Trigger: Fire or inappropriate grazing management can eliminate the squirreltail understory and transition to community phase 4.1 or 4.2.
Slow variable: Increased seed production and cover of annual non-native species.
Threshold: Increased, continuous fine fuels modify the fire regime by changing intensity, size and spatial variability of fires. Changes in plant community composition and spatial variability of vegetation due to the loss of perennial bunchgrasses and big sagebrush truncate energy capture and impact the nutrient cycling and distribution.
T3B: Transition from Shrub State 3.0 to Tree State 5.0:
Trigger: Time and lack of disturbance allows for maturation of the tree community. This may be coupled with grazing management that favors shrub and tree growth.
Slow variable: Over time, with lack of fire, the abundance and size of trees increase.
Threshold: Trees overtop mountain big sagebrush and out-compete shrubs for water and sunlight. Shrub skeletons exceed live shrubs with minimal recruitment of new cohorts. Bare ground areas are large and connected.
R3A: Restoration from Shrub State 3.0 to Seeded State 6.0:
Brush management and seeding of crested wheatgrass and/or other desired species. Presence of non-native annual species will make this restoration pathway difficult.
Annual State 4.0:
This state has two community phases, both characterized by a dominance of non-native annual grasses and forbs. Shrub cover is present in one phase, while the other is primarily annual grasses. Sagebrush and/or rabbitbrush may dominate the overstory. Annual non-native species dominate the understory. Ecological dynamics are significantly altered in this state. Annual non-native species create a highly combustible fuel bed that shortens the fire return interval. Nutrient cycling is spatially and temporally truncated as annual plants contribute significantly less to deep soil carbon. Because this is a productive site, some deep-rooted perennial grasses may remain, even in the annual state. Without management, it is unlikely these plants will be able to recruit in the presence of dominant annual grasses.
Community Phase 4.1:
Annual non-native plants such as cheatgrass dominate the site. This phase may have seeded species present if resulting from a failed seeding attempt. Needlegrasses, mountain big sagebrush, and other shrubs are only present in trace amounts and may be missing from the community.
Community Phase Pathway 4.1a, from phase 4.1 to phase 4.2:
Time and lack of disturbance allows for shrubs to reestablish. Probability of sagebrush reestablishment is extremely low.
Community Phase 4.2:
Annual non-native species, primarily cheatgrass, dominate the understory. Sprouting shrubs dominate the overstory. Perennial bunchgrasses are a minor component or missing. Seeded species may be present.
Community Phase Pathway 4.2a, from phase 4.2 to 4.1:
Fire kills non-sprouting shrubs and allows annual non-native species to dominate site.
Tree State 5.0:
This state is characterized by a dominance of pinyon and/or juniper in the overstory. It occurs where sagebrush sites exist adjacent to stands of trees. Big sagebrush and perennial bunchgrasses may still be present, but they are no longer controlling site resources. Skeletons of dead sagebrush plants are apparent. Soil moisture, soil nutrients, soil organic matter distribution and nutrient cycling have been spatially and temporally altered.
Community Phase 5.1:
Pinyon and juniper dominate. Trees are actively growing with noticeable leader growth. Mountain big sagebrush is stressed and dying. Needlegrass and other perennial grasses reduced. Annual non-native species are present under tree canopies. Bare ground interspaces are large and connected.
Community Phase Pathway 5.1a, from phase 5.1 to 5.2:
Absence of disturbance over time allows for tree cover and density to further increase and out-compete the herbaceous understory species for sunlight and water.
Community Phase 5.2:
Pinyon and/or juniper dominate the site and tree leader growth is minimal. Annual non-native species may be the dominant understory species and will typically be found under tree canopies. Trace amounts of sagebrush may be present, however dead skeletons will be more numerous than living sagebrush. Bunchgrass may or may not be present. Bare ground areas are large and connected, and soil redistribution is apparent.
Community Phase Pathway 5.2a, from phase 5.2 to 5.1:
Tree thinning treatment (typically for fuels management) removes some tree cover and may allow sagebrush to survive. Without further management this pathway is temporary.
T5A: Transition from Tree State 5.0 to Annual State 4.0:
Trigger: Catastrophic crown fire would reduce or eliminate trees to transition the site to 4.1. Tree removal when annual non-natives such as cheatgrass are present would also transition the site to state 4.0.
Slow variable: Increased seed production and cover of annual non-native species.
Threshold: Increased, continuous fine fuels modify the fire regime by changing intensity, size and spatial variability of fires. Changes in plant community composition and spatial variability of vegetation due to the loss of perennial bunchgrasses and sagebrush truncate energy capture and impact the nutrient cycling and distribution.
R5A: Restoration from Tree State 5.0 to Shrub State 6.0:
Tree removal with minimum soil disturbance such as hand felling or mastication within community phase 5.1 when native grasses are still present.
R5B: Restoration from Tree State 5.0 to Seeded State 6.0:
Tree removal and seeding of desired species. Tree removal practices that minimize soil disturbance are recommended. Probability of success declines with increased presence of non-native annual species.
Seeded State 6.0:
This state has two community phases: a grass-dominated phase and a shrub dominated phase. This state is characterized by the dominance of seeded introduced wheatgrass species in the understory. Crested wheatgrass is a dominant plant in this phase. Conservation practices such as brush management and prescribed grazing should be used to maintain the perennial bunchgrasses and other desirable species.
Community Phase 6.1:
Seeded wheatgrasses and/or other seeded species dominate the community. Non-native annual species are present. Trace amounts of mountain big sagebrush may be present, especially if seeded.
Community Phase Pathway 6.1a, from phase 6.1 to 6.2:
Time and lack of disturbance allows shrubs to dominate. This process may be accelerated through inappropriate grazing management.
Community Phase 6.2:
Mountain big sagebrush and/or bitterbrush increases and dominates the overstory. Seeded wheatgrass species are a minor component. Annual non-native species may be present in trace amounts. Pinyon and/or juniper may be present.
Community Phase Pathway 6.2a, from phase 6.2 to 6.1:
Fire, brush management and/or Aroga moth infestation reduces sagebrush overstory and allows for seeded wheatgrasses or other seeded grasses to increase.
T6A: Transition from Seeded State 6.0 to Annual State 4.0:
Trigger: Catastrophic fire.
Slow variables: Increased production and cover of non-native annual species.
Threshold: Increased continuous fine fuels modify the fire regime by changing intensity, size, and spatial variability of fires. Changes in plant community composition and spatial variability of vegetation due to the loss of perennial bunchgrasses and sagebrush truncate energy capture spatially and temporally, thus impacting nutrient cycling and distribution.
T6B: Transition from Seeded State 6.0 to Tree State 5.0:
Trigger: Time and lack of disturbance or management action allows for pinyon and/or juniper to dominate. This may be coupled with grazing management that favors tree establishment by reducing understory herbaceous competition for site resources.
Slow variables: Over time, the abundance and size of trees will increase.
Threshold: Trees dominate ecological processes and number of shrub skeletons exceed number of live shrubs.
State and transition model
More interactive model formats are also available.
View Interactive Models
Click on state and transition labels to scroll to the respective text
Ecosystem states
State 1 submodel, plant communities
State 1
Reference State
Community 1.1
Reference Plant Community
The reference plant community is dominated by western, Columbia and/or Letterman needlegrasses, mountain big sagebrush and antelope bitterbrush. Potential vegetative composition is about 65% grasses, 10% forbs and 25% shrubs. Approximate ground cover (basal and crown) is 30 to 45 percent. Bare ground is approximately 35%, surface rock fragments are approximately 25%, shrub canopy is 15 to 25% and foliar cover for perennial herbaceous plants is 40 to 60%. Dead branches within individual shrubs are common and standing dead shrub canopy material may be as much as 15% of total woody canopy. Some of the mature bunchgrasses (less than 10%) have dead centers. Within plant interspaces litter is approximately 35% cover and the depth of litter is approximately one-half inch.
Figure 4. Annual production by plant type (representative values) or group (midpoint values)
Table 5. Annual production by plant type
| Plant type | Low (lb/acre) |
Representative value (lb/acre) |
High (lb/acre) |
|---|---|---|---|
| Grass/Grasslike | 650 | 975 | 1300 |
| Shrub/Vine | 250 | 375 | 500 |
| Forb | 100 | 150 | 200 |
| Total | 1000 | 1500 | 2000 |
Additional community tables
Table 6. Community 1.1 plant community composition
| Group | Common name | Symbol | Scientific name | Annual production (lb/acre) | Foliar cover (%) | |
|---|---|---|---|---|---|---|
|
Grass/Grasslike
|
||||||
| 1 | Primary Perennial Grasses | 660–1140 | ||||
| Letterman's needlegrass | ACLE9 | Achnatherum lettermanii | 175–225 | – | ||
| Columbia needlegrass | ACNEN2 | Achnatherum nelsonii ssp. nelsonii | 175–225 | – | ||
| western needlegrass | ACOCO | Achnatherum occidentale ssp. occidentale | 175–225 | – | ||
| basin wildrye | LECI4 | Leymus cinereus | 75–225 | – | ||
| muttongrass | POFE | Poa fendleriana | 30–120 | – | ||
| mountain brome | BRMA4 | Bromus marginatus | 30–120 | – | ||
| 2 | Secondary Perennial Grasses | 75–225 | ||||
| Thurber's needlegrass | ACTH7 | Achnatherum thurberianum | 8–45 | – | ||
| sedge | CAREX | Carex | 8–45 | – | ||
| squirreltail | ELEL5 | Elymus elymoides | 8–45 | – | ||
| needle and thread | HECO26 | Hesperostipa comata | 8–45 | – | ||
| spike fescue | LEKI2 | Leucopoa kingii | 8–45 | – | ||
| purple oniongrass | MESP | Melica spectabilis | 8–45 | – | ||
| Sandberg bluegrass | POSE | Poa secunda | 8–45 | – | ||
|
Forb
|
||||||
| 3 | Perennial | 75–225 | ||||
| spike fescue | LEKI2 | Leucopoa kingii | 8–45 | – | ||
| purple oniongrass | MESP | Melica spectabilis | 8–45 | – | ||
| arrowleaf balsamroot | BASA3 | Balsamorhiza sagittata | 8–45 | – | ||
| tapertip hawksbeard | CRAC2 | Crepis acuminata | 8–45 | – | ||
| lupine | LUPIN | Lupinus | 8–45 | – | ||
|
Shrub/Vine
|
||||||
| 4 | Primary Shrubs | 225–375 | ||||
| mountain big sagebrush | ARTRV | Artemisia tridentata ssp. vaseyana | 150–225 | – | ||
| antelope bitterbrush | PUTR2 | Purshia tridentata | 75–150 | – | ||
| sedge | CAREX | Carex | 8–45 | – | ||
| 5 | Secondary Shrubs | 75–150 | ||||
| Forb, perennial | 2FP | Forb, perennial | 51–90 | – | ||
| arrowleaf balsamroot | BASA3 | Balsamorhiza sagittata | 8–45 | – | ||
| tapertip hawksbeard | CRAC2 | Crepis acuminata | 8–45 | – | ||
| lupine | LUPIN | Lupinus | 8–45 | – | ||
| Utah serviceberry | AMUT | Amelanchier utahensis | 8–30 | – | ||
| yellow rabbitbrush | CHVI8 | Chrysothamnus viscidiflorus | 8–30 | – | ||
| mormon tea | EPVI | Ephedra viridis | 8–30 | – | ||
| slender buckwheat | ERMI4 | Eriogonum microthecum | 8–30 | – | ||
| currant | RIBES | Ribes | 8–30 | – | ||
| snowberry | SYMPH | Symphoricarpos | 8–30 | – | ||
|
Tree
|
||||||
| 6 | Evergreen | 16–60 | ||||
| mountain big sagebrush | ARTRV | Artemisia tridentata ssp. vaseyana | 150–225 | – | ||
| mormon tea | EPVI | Ephedra viridis | 8–30 | – | ||
| Utah juniper | JUOS | Juniperus osteosperma | 8–30 | – | ||
| singleleaf pinyon | PIMO | Pinus monophylla | 8–30 | – | ||
Interpretations
Animal community
Despite low palatability, mountain big sagebrush is eaten by sheep, cattle, goats, and horses. Chemical analysis indicates that the leaves of big sagebrush equal alfalfa meal in protein, have a higher carbohydrate content, and yield twelvefold more fat (USDA-Forest Service 1937). Many wildlife species are dependent on the sagebrush ecosystem including the greater sage grouse, sage sparrow, pygmy rabbit and the sagebrush vole. Dobkin and Sauder (2004) identified 61 species, including 24 mammals and 37 birds, associated with the shrub-steppe habitats of the Intermountain West.
Mountain big sagebrush sites provide nesting, brood-rearing, and fall and winter habitat for sage grouse (Centrocercus urophasianus). Sage grouse require sagebrush for food and cover during each stage of their life cycle. The abundance and diversity of perennial forbs and grasses provides important food for hens during the pre-laying period and comprise more than half of the juvenile diet until the broods are approximately three months old (McAdoo and Back 2001).
Antelope bitterbrush is an important shrub species to a variety of animals, such as domestic livestock, antelope, deer, and elk. Bitterbrush is critical browse for mule deer, as well as domestic livestock, antelope, and elk (Wood 1995, Clements and Young 2002). Antelope bitterbrush is most commonly found on soils which provide minimal restriction to deep root penetration, such as coarse textured soil, or finer textured soil with high stone content (Driscoll 1964). Grazing tolerance of antelope bitterbrush is dependent on site conditions (Garrison 1953).
The three primary needlegrass species found on this site, Letterman’s, Columbia, and western, are common in cooler, moist, higher elevation areas of the sagebrush biome, and are considered to act as increasers with heavy grazing (Tisdale and Hironaka 1981, Hironaka and Fosberg 1979, Monsen et al. 2004). This may be because all grasses become less palatable when mature, due to their characteristic hardened pointed calluses. While Letterman’s needlegrass provides forage for both livestock and wildlife (Parker 1975), it is only rated as fair for cattle and poor for sheep (USDA Forest Service 1937). It begins growth early in the year and is available to be utilized when other grasses are not yet palatable. This plant is especially important fall forage for big game (Monsen et al. 2004). Letterman’s needlegrass has been shown to increase under long-term sheep grazing (Ellison 1954). There is some evidence that it decreases under cattle and horse grazing (Bowns and Bagley 1986).
Columbia needlegrass is a good forage source in the spring and summer, but livestock tend to avoid it as the seeds mature; the pointed callus in the mature inflorescence sometimes becomes injurious (Monsen et al. 2004). This grass recovers well with rest from grazing (Monsen et al. 2004).
The early growth and abundant production of basin wildrye make it a valuable source of forage for livestock. It is important forage for cattle and is readily grazed by cattle and horses in early spring and fall. Though coarse-textured during the winter, basin wildrye may be utilized more frequently by livestock and wildlife when snow has covered low shrubs and other grasses. Basin wildrye is used often as a winter feed for livestock and wildlife; not only providing roughage above the snow but also cover in the early spring months (Majerus 1992). Inadequate rest and recovery from defoliation causes a decrease in basin wildrye and an increase in sagebrush and rubber rabbitbrush (Young et al. 1976, Roundy 1985). Spring defoliation of basin wildrye and/or consistent, heavy grazing during the growing season has been found to significantly reduce basin wildrye production and density (Krall et al. 1971). Additionally, native basin wildrye suffers from low seed viability and low seedling vigor (Young and Evans 1981). Roundy (1985) found that although basin wildrye is adapted to seasonally dry soils, high and frequent spring precipitation is necessary to establish it from seed. This suggests that establishment of basin wildrye seedlings occurs only during years of unusually high precipitation. Therefore, reestablishment of a stand may be episodic.
Bottlebrush squirreltail generally increases in abundance when moderately grazed or protected (Hutchings & Stewart, 1953). Muttongrass (Poa fendleriana), a minor component on this ecological site, is relatively grazing tolerant. It is palatable and nutritional forage for livestock and wildlife when it is in the early stages of growth. It rates as excellent forage for cattle and horses, and good for sheep, elk and deer (Dayton 1937). Muttongrass persists well in open areas and under canopies of oak and other shrubs (Monsen et al. 2004). Muttongrass may be more shade tolerant than other perennial bunchgrasses and may persist in the understory as the canopy closes (Erdman 1970).
Overgrazing leads to an increase in big sagebrush and a decline in understory plants like basin wildrye. Grasses like squirreltail and bluegrasses (Poa fendleriana and P. secunda) are more tolerant of grazing and can become dominant. Bluegrass species are less common on certain sites in this group. Reduced bunchgrass vigor or density also provides an opportunity for cheatgrass and other invasive species to occupy interspaces. Over time, this leads to increased fire frequency and potentially an annual plant community. This site is likely to see an increase in shrubs and will have significant bare ground in the interspaces understory plant health declines.
Hydrological functions
Rills and water flow patterns are typically non-existent. Water flow patterns may rarely be observed on steeper slopes in areas recently subjected to summer convection storms or rapid spring snowmelt. Pedestals are none to rare. Occurrence is usually limited to areas of water flow patterns. Frost heaving of shallow rooted plants should not be considered a normal condition. Fine litter (foliage from grasses and annual and perennial forbs) are expected to move the distance of slope length during intense summer convection storms or rapid snowmelt events. Persistent litter (large woody material) will remain in place except during catastrophic events. Perennial herbaceous plants (especially deep-rooted bunchgrasses [i.e. needlegrasses and basin wildrye] slow runoff and increase infiltration. Shrub canopy and associated litter break raindrop impact and provide opportunity for snow catch and accumulation on site.
Recreational uses
Aesthetic value is derived from the diverse floral and faunal composition and the colorful flowering of wild flowers and shrubs during the spring and early summer. This site offers rewarding opportunities to photographers and for nature study. This site is used for camping and hiking and has potential for upland and big game hunting.
Other products
Native Americans used big sagebrush leaves and branches for medicinal teas, and the leaves as a fumigant. Bark was woven into mats, bags and clothing. Basin wildrye was used as bedding for various Native American ceremonies, providing a cool place for dancers to stand.
Other information
Antelope bitterbrush has been used extensively in land reclamation. Antelope bitterbrush enhances succession by retaining soil and depositing organic material and in some habitats and with some ecotypes, by fixing nitrogen. Letterman’s needlegrass has been used successfully in revegetating mine spoils. This species also has good potential for erosion control. Basin wildrye is useful in mine reclamation, fire rehabilitation and stabilizing disturbed areas. Its usefulness in range seeding, however, may be limited by initially weak stand establishment. Mountain brome is an excellent native bunchgrass for seeding alone or in mixtures in disturbed areas, including depleted rangelands, burned areas, roadways, mined lands, and degraded riparian zones.
Supporting information
Type locality
| Location 1: Carson City County, NV | |
|---|---|
| General legal description | This site also occurs in Douglas, Lyon, Mineral, Storey and Washoe counties, Nevada. |
Other references
Akinsoji, A. 1988. Postfire vegetation dynamics in a sagebrush steppe in southeastern Idaho, USA. Vegetation 78:151-155.
Bates, J. D., T. Svejcar, R. F. Miller, and R. A. Angell. 2006. The effects of precipitation timing on sagebrush steppe vegetation. Journal of Arid Environments 64:670-697.
Baughman, O. W., R. Burton, M. Williams, P. J. Weisberg, T. E. Dilts, and E. A. Leger. 2017. Cheatgrass die-offs: a unique restoration opportunity in northern Nevada. Rangelands 39(6):165-173.
Baughman, O. W., S. E. Meyer, Z. T. Aanderud, and E. A. Leger. 2016. Cheatgrass die-offs as an opportunity for restoration in the Great Basin, USA: Will local or commercial native plants succeed where exotic invaders fail? Journal of Arid Environments 124:193-204.
Bentz, B., D. Alston, and T. Evans. 2008. Great Basin insect outbreaks. Pages 45-48 in Collaborative Management and Research in the Great Basin -- Examining the issues and developing a framework for action U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, CO.
Bich, B. S., J. L. Butler, and C. A. Schmidt. 1995. Effects of differential livestock use on key plant species and rodent populations within selected oryzopsis hymenoides/Hilaria jamesii communities of Glen Canyon National Recreation Area. The Southwestern Naturalist 40:281-287.
Blaisdell, J. P. and W. F. Mueggler. 1956. Sprouting of bitterbrush (Purshia tridentata) following burning or top removal. Ecology 37:365-370.
Blaisdell, J.P. 1953. Ecological effects of planned burning of sagebrush-grass range on the Upper Snake River Plains. Tech. Bull. 1975. Washington, DC: U.S. Department of Agriculture. 39 p.
Blaisdell, J.P. R.B. Murray, and E.D. McArthur. 1982. Managing Intermountain rangelands-- sagebrush-grass ranges. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Gen. Tech. Rep. INT-134. 41 p.
Blank, R. R., and J. A. Young. 1998. Heated substrate and smoke: Influence on seed emergence and plant growth. Journal of Range Management 51(5):577-583.
Blank, R. R., C. Clements, T. Morgan, D. Harmon, and F. Allen. 2020. Suppression of cheatgrass by perennial bunchgrasses. Rangeland Ecology & Management 73(6):766-771.
Booth, D. T., C. G. Howard, and C. E. Mowry. 2006. 'Nezpar' Indian ricegrass: description, justification for release, and recommendations for use. Rangelands Archives 2:53-54.
Bowns, J. E., Bagley, C. F. 1986. Vegetation responses to long term sheep grazing on mountain ranges. Journal of Range Management, 39(5).
Bradford, J. B. and W. K. Lauenroth. 2006. Controls over invasion of Bromus tectorum: The importance of climate, soil, disturbance and seed availability. Journal of Vegetation Science 17(6): 693-704.
Brehm, J. R. 2019. Cheatgrass die-off in the Great Basin: A comparison of remote sensing detection methods and identification of environments favorable to die-off. M.S. Thesis. University of Nevada, Reno.
Britton, C. M., G. R. McPherson, and F. A. Sneva. 1990. Effects of burning and clipping on five bunchgrasses in eastern Oregon. Great Basin Naturalist 50(2):115-120.
Brooks, M. L., C. M. D'Antonio, D. M. Richardson, J. B. Grace, J. E. Keeley, J. M. Ditomaso, R. J. Hobbs, M. Pellant, and D. Pyke. 2004. Effects of Invasive Alien Plants on Fire Regimes. BioScience 54(7):677-688.
Brown, J. K., and J. K. Smith, editors. 2000. Wildland Fire in Ecosystems: Effects of Fire on Flora. Gen. Tech. Rep. RMRS-GTR-42 vol. 2. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Ogden, UT. 257 p.
Bunting, S.C., B.M. Kilgore, and C.L. Bushey. 1987. Guidelines for prescribed burning sagebrush/grass rangelands in the northern Great Basin. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. Gen. Tech. Rep. INT-231.33 p.
Burkhardt, J.W. and E.W. Tisdale. 1969. Nature and successional status of western juniper vegetation in Idaho. Journal of Range Management 22(4):264-270.
Busse, D., A. Simon, and M. Riegel. 2000. Tree-growth and understory responses to low-severity prescribed burning in thinned Pinus ponderosa forests of central Oregon. Forest Science 46:258-268.
Butler, M., F. Brummer, J. Weber, and R. Simmons. 2011. Restoring Central Oregon Rangeland from Ventenata and Medusahead to a Sustainable Bunchgrass Environment – Warm Springs and Ashwood. Central Oregon Agriculture Research and Extension Center.
Caudle, D., J. DiBenedetto, M. Karl, H. Sanchez, and C. Talbot. 2013. Interagency Ecological Site Handbook for Rangelands. Available at: http://jornada.nmsu.edu/sites/jornada.nmsu.edu/files/InteragencyEcolSiteHandbook.pdf. Accessed 4 October 2013.
Chambers, J. C., B. A. Roundy, R. R. Blank, S. E. Meyer, and A. Whittaker. 2007. What makes great basin sagebrush ecosystems invasible by Bromus tectorum? Ecological Monographs 77:117-145.
Chambers, J., B. Bradley, C. Brown, C. D’Antonio, M. Germino, J. Grace, S. Hardegree, R. Miller, and D. Pyke. 2013. Resilience to stress and disturbance, and resistance to Bromus tectorum L. invasion in cold desert shrublands of western North America. Ecosystems:1-16.
Chambers, J.C., B.A. Roundy, R.R. Blank, S.E. Meyer, and A. Whittaker. 2007. What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecological Monographs 77:117-145.
Clark, R. G., M. B. Carlton, and F. A. Sneva. 1982. Mortality of bitterbrush after burning and clipping in Eastern Oregon. Journal of Range Management 35:711-714.
Clements, C. D. and J. A. Young. 2002. Restoring antelope bitterbrush. Rangelands 24:3-6.
Clements, C. D., D. N. Harmon, R. R. Blank, and M. Weltz. 2017. Improving seeding success on cheatgrass-infested rangelands in northern Nevada. Rangelands 39(6):174-181.
Comstock, J.P. and J.R. Ehleringer. 1992. Plant adaptation in the Great Basin and Colorado Plateau. The Great Basin Naturalist 52:195-215.
Cook, C. W. 1962. An evaluation of some common factors affecting utilization of desert range species. Journal of Range Management 15:333-338.
Cook, C. W. and R. D. Child. 1971. Recovery of Desert Plants in Various States of Vigor. Journal of Range Management 24:339-343.
Cook, J. G., T. J. Hershey, and L. L. Irwin. 1994. Vegetative Response to Burning on Wyoming Mountain-Shrub Big Game Ranges. Journal of Range Management 47:296-302.
Crawford, J.A., R.A. Olson, N.E. West, J.C. Mosley, M.A. Schroeder, T.D. Whitson, R.F. Miller, M.A. Gregg, and C.S. Boyd. 2004. Ecology and management of sage-grouse and sage-grouse habitat. Journal of Range Management. 57: 2-19.
Cronquist, A., A. H. Holmgren, N. H. Holmgren, J. L. Reveal, & P. K. Holmgren 1977. Intermountain flora. Vol. 6. The New York Botanical Garden. Columbia University Press, New York, New York.
D'Antonio, C. M., and P. M. Vitousek. 1992. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review of Ecology and Systematics 23:63-87.
Davies, K. W., and T. J. Svejcar. 2008. Comparison of medusahead-invaded and noninvaded Wyoming big sagebrush steppe in southeastern Oregon. Rangeland Ecology and Management 61(6):623-629.
Davies, K. W., C. S. Boyd, D. D. Johnson, A. M. Nafus, and M. D. Madsen. 2015. Success of seeding native compared with introduced perennial vegetation for revegetating medusahead-invaded sagebrush rangeland. Rangeland Ecology & Management 68(3):224-230.
Dayton, W. 1937. Range plant handbook. USDA, Forest Service. Bull.
Dittberner, P.L., Olsen, M.R. 1983. The Plant Information Network (PIN) database: Colorado, Montana, North Dakota, Utah, and Wyoming. FWS/OBS-83/36. Washington, DC: U.S. Department of Interior, Fish and Wildlife Service, Division of Biological Services, Research and Development, Western Energy and Land Use Team. 786 P.
Dobkin, D.S. and J.D. Sauder. 2004. Shrub steppe landscapes in jeopardy. Distributions, abundances, and the uncertain future of birds and small mammals in the Intermountain West. Bend, Oregon. USA: High Desert Ecological Research Institute.
Dobrowolski, J.P., Caldwell, M.M. and Richards, J.H. 1990. Basin hydrology and plant root systems. In: Plant Biology of the Basin and Range. New York, NY: Springer-Verlag Pub.
Driscoll, R. S. 1964. A relict area in the central Oregon juniper zone. Ecology 45:345-353.
Ellison, L. 1954. Subalpine vegetation of the Wasatch Plateau, Utah. Ecological Monographs 24(2):89-184.
Erdman, J. A. 1970. Pinyon-juniper succession after natural fires on residual soils of Mesa Verde, Colorado. Brigham Young University Science Bulletin-Biological Series 11:1-26.
Furbush, P. (1953). Control of Medusa-Head on California Ranges. Journal of Forestry 51(2): 118-121.
Furniss, M. M. and W. F. Barr. 1975. Insects affecting important native shrubs of the northwestern United States. US Intermountain Forest and Range Experiment Station. USDA Forest Service General Technical Report INT INT-19.
Ganskopp, D. 1988. Defoliation of Thurber needlegrass: herbage and root responses. Journal of Range Management 41(6):472-476.
Garrison, G. A. 1953. Effects of clipping on some range shrubs. Journal of Range Management 6:309-317.
Harris, G. A. 1967. Some Competitive Relationships between Agropyron spicatum and Bromus tectorum. Ecological Monographs 37(2): 89-111.
Hironaka, M. 1994. Medusahead: natural successor to the cheatgrass type in the northern Great Basin. Pages 89-91 in Proceedings of Ecology and Management of Annual Rangelands. Gen. Tech. Report INT-313. USDA Forest Service, Intermountain Research Station., Boise, ID.
Hironaka, M., and E. W. Tisdale. 1973. Growth and development of Sitanion hystrix and Poa sandbergii. Research Memorandum, RM 73-16. U.S. International Biological Program, Desert Biome.
Houston, D.B. 1973. Wildfires in northern Yellowstone National Park. Ecology 54(5):1111-1117.
Kerns, B. K., W. G. Thies, and C. G. Niwa. 2006. Season and severity of prescribed burn in ponderosa pine forests: implications for understory native and exotic plants. Ecoscience 13:44-55.
Kitchen, S. G. and E. D. McArthur. 2007. Big and black sagebrush landscapes. In: Fire ecology and mangement of the major ecosystems of southern Utah. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. General Technical Report RMRS-GTR-202. p 73-95.
Klemmedson, J. O. and J. G. Smith. 1964. Cheatgrass (Bromus Tectorum L.). The botanical review 30(2): 226-262.
Koniak, S. 1985. Succession in piñon-juniper woodlands following wildfire in the Great Basin. The Great Basin Naturalist 45(3):556-566.
Krall, J. L., J. R. Stroh, C. S. Cooper, and S. R. Chapman. 1971. Effect of time and extent of harvesting basin wildrye. Journal of Range Management 24:414-418.
Krall, J. L., J. R. Stroh, C. S. Cooper, and S. R. Chapman. 1971. Effect of time and extent of harvesting basin wildrye. Journal of Range Management 24:414-418.
Kuntz, D.E. 1982. Plant response following spring burning in an Artemisia tridentata subsp. vaseyana/Festuca idahoensis habitat type. Moscow, ID: University of Idaho. 73 p. Thesis.
Mack, R. N. and D. Pyke. 1983. The demography of Bromus Tectorum: Variation in time and space. Journal of Ecology 71(1): 69-93.
Majerus, M. E. 1992. High-stature grasses for winter grazing. Journal of soil and water conservation 47:224-225.
Majerus, M. E. 1992. High-stature grasses for winter grazing. Journal of soil and water conservation 47:224-225.
Mangla, S., R. Sheley, and J. J. James. 2011. Field growth comparisons of invasive alien annual and native perennial grasses in monocultures. Journal of Arid Environments 75(2):206-210.Monsen, S. B. 1992. The competitive influences of cheatgrass (Bromus tectorum) on site restoration. Pages 43-50 in Proceedings - Ecology, Management, and Restoration of Intermountain Annual Rangelands. General Technical Report INT-GTR-313. U.S.D.A Forest Service Intermountain Research Station, Boise, ID.
McAdoo, J.K. and G.N. Back. 2001. Sage Grouse Biology, University of Nevada Reno, Extension FS-01-43.
McArthur, E. D., et al. 1982. Characteristics and hybridization of important Intermountain shrubs: 3. Sunflower family. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Research Paper INT-177 43(5).
McConnell, B. R. 1961. Notes on some rooting characteristics of antelope bitterbrush. Research Note No. 204. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 5 p.
McConnell, B. R. and J. G. Smith. 1977. Influence of grazing on age-yield Interactions in bitterbrush. Journal of Range Management 30:91-93.
Miller, R.F., J.C. Chambers, D.A. Pyke, F.B. Pierson, and C.J. Williams. 2013. A review of fire effects on vegetation and soils in the Great Basin region: response and ecological site characteristics. Gen. Tech. Rep. RMRS-GTR-308. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 126 p.
Miller, R.F., T.J. Svejcar, and J.A. Rose. 2000. Impacts of western juniper on plant community composition and structure. Journal of Range Management 53(6):574-585.
Monaco, T. A., Charles T. Mackown, Douglas A. Johnson, Thomas A. Jones, Jeanette M. Norton, Jay B. Norton, and Margaret G. Redinbaugh. 2003. Nitrogen effects on seed germination and seedling growth. Journal of Range Management 56(6):646-653.
Monsen, S. B., R. Stevens, and N. L. Shaw. 2004. Grasses. Pages 295-424 in Restoring western ranges and wildlands, vol. 2. Gen. Tech. Rep. RMRS-GTR-136-vol-2. USDA: Forest Service, Rocky Mountain Research Station, Fort Collins, CO.
Monsen, S.B., R. Stevens, N.L. Shaw, comps. 2004. Restoring western ranges and wildlands. Gen. Tech. Rep. RMRS-GTR-136-vol-2. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. Pages 295-698 plus index.
Murray, R. 1983. Response of antelope bitterbrush to burning and spraying in southeastern Idaho. Tiedemann, Arthur R.; Johnson, Kendall L., compilers. Research and management of bitterbrush and cliffrose in western North America. Ogden, UT: US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. General Technical Report INT-152. p 142-152.
Neuenschwander, L.F. 1980. Broadcast burning of sagebrush in the winter. Journal of Range Management (33)3:233-236.
Noste, N.V. and C.L. Bushey. 1987. Fire response of shrubs of dry forest habitat types in Montana and Idaho. Gen. Tech. Rep. INT-239. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 22 p.
Noy-Meir, I. 1973. Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics 4:25-51.
Ogle, D.G., Tilley, D., and L. St. John. 2012. Plant Guide for basin wildrye (Leymus cinereus). USDA-Natural Resources Conservation Service, Aberdeen Plant Materials Center. Aberdeen, Idaho 83210.
Parker, K. G. 1975. Some important Utah range plants. Extension Service Bulletin EC-383. Logan, UT: Utah State University. 174 p.
Patton, B. D., Hironaka, M., and Bunting, S. C. 1988. Effect of burning on seed production of bluebunch wheatgrass, Idaho fescue, and Columbia needlegrass. Journal of Range Management, 41, 232-234.
Pearson, L. 1964. Effect of harvest date on recovery of range grasses and shrubs. Agronomy Journal 56:80-82.
Pearson, L. C. 1965. Primary production in grazed and ungrazed desert communities ofeastern Idaho. Ecology 46:278-285.
Pellant, M. and C. Hall. 1994. Distribution of two exotic grasses in intermountain rangelands: status in 1992, USDA Forest Service Gen. Tech Report INT-GTR-313S: 109-112.
Perryman, B.L. and Q.D. Skinner. 2007. A Field Guide to Nevada Grasses. Indigenous Rangeland Management Press, Lander, Wyoming. 256 p.
Quinones, F.A. 1981. Indian ricegrass evaluation and breeding. Bulletin 681. Las Cruces, NM: New Mexico State University, Agricultural Experiment Station. 19 p.
Reynolds, T.D., and L. Fraley Jr. 1989. Root profiles of some native and exotic plant species in southeastern Idaho. Environmental and Experimental Botany. 29(2): 241-248.
Richards, J. H. and M. M. Caldwell. 1987. Hydraulic lift: substantial nocturnal water transport between soil layers by Artemisia tridentata. Oecologia. 73(4): 486-489.
Roundy, B. A. 1985. Emergence and establishment of basin wildrye and tall wheatgrass in relation to moisture and salinity. Journal of Range Management 38:126-131.
Roundy, B. A. 1985. Emergence and establishment of basin wildrye and tall wheatgrass in relation to moisture and salinity. Journal of Range Management 38:126-131.
Schlatterer, E.F. 1972. Plant communities found in the Sawtooth, White Goud, Boulder and Pioneer Mountains. USDA Forest Serv., Intermtn. Reg. (mimeo) 111 pp.
Sheley, R. L., E. A. Vasquez, A. Chamberlain, and B. S. Smith. 2012. Landscape-scale rehabilitation of medusahead (Taeniatherum caput-medusae)-dominated sagebrush steppe. Invasive Plant Science and Management 5(4):436-442.
Smith, M.A. and F. Busby. 1981. Prescribed burning: effective control of sagebrush in Wyoming. RJ-165. Laramie, WY: University of Wyoming, Agricultural Experiment Station. 12 p.
Stubbendieck, J., J.T. Nichols, and K.K. Roberts. 1985. Nebraska range and pasture grasses (including grass-like plants). E.C. 85-170. Lincoln, NE: University of Nebraska, Department of Agriculture, Cooperative Extension Service. 75 p.
Tisdale, E. W.; Hironaka, M. 1981. The sagebrush-grass region: a review of the ecological literature. Bull. 33. Moscow, ID: University of Idaho, Forest, Wildlife and Range Experiment Station. 31 p.
Turner, G. T. 1969. Responses of mountain grassland vegetation to gopher control, reduced grazing, and herbicide. Journal of Range Management, 22:377-383.
USDA Forest Service. 1937. Range Plant Handbook. Dover Publications, New York. 816 p.
Vollmer, J. L. and J. G. Vollmer (2008). Controlling cheatgrass in winter range to restore habitat and endemic fire Proceedings-Shrublands under fire: disturbance and recovery in a changing world. RMRS-P-52., Cedar City, UT, Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station.
Weisberg, P. J., T. E. Dilts, O. W. Baughman, S. E. Meyer, E. A. Leger, K. J. Van Gunst, and L. Cleeves. 2017. Development of remote sensing indicators for mapping episodic die-off of an invasive annual grass (Bromus tectorum) from the Landsat archive. Ecological Indicators 79:173-181.
Whisenant, S. G. 1999. Repairing Damaged Wildlands: a process-orientated, landscape-scale approach (Vol. 1). Cambridge, UK: Cambridge University Press. 312 p.
Williams, J.R., Morris, L.R., Gunnell, K.L., Johanson, J.K. and Monaco, T.A., 2017. Variation in sagebrush communities historically seeded with crested wheatgrass in the eastern Great Basin. Rangeland Ecology & Management, 70(6):683-690.
Wood, M. K., Bruce A. Buchanan, & William Skeet. 1995. Shrub preference and utilization by big game on New Mexico reclaimed mine land. Journal of Range Management 48:431-437.
Wright, H. A. 1971. Why squirreltail is more tolerant to burning than needle-and-thread. Journal of Range Management 24:277-284.
Young, J. A., and R. A. Evans. 1977. Squirreltail seed germination. Journal of Range Management 30(1):33-36.
Young, J. A., R. A. Evans, and P. T. Tueller. 1976. Great Basin plant communities-pristine and grazed. Holocene environmental change in the Great Basin. Nevada Archeological Survey Research Paper 6:186-215.
Young, R.P. 1983. Fire as a vegetation management tool in rangelands of the Intermountain Region. In: Monsen, S.B. and N. Shaw (compilers). Managing Intermountain rangelands-- improvement of range and wildlife habitats: Proceedings; 1981 September 15-17; Twin Falls, ID; 1982 June 22-24; Elko, NV. Gen. Tech. Rep. INT-157. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. p 18-31.
Zschaechner, G. A. 1984. Studying rangeland fire effects: a case study in Nevada. Pages 66-84 in Rangeland Fire Effects: A Symposium. November 27-29, 1984 in Boise, ID. USDI-BLM Idaho State Office, Boise, ID.
Contributors
DK/FR/GKB
Tamzen Stringham
Patti Novak-Echenique
Approval
Kendra Moseley, 4/10/2024
Rangeland health reference sheet
Interpreting Indicators of Rangeland Health is a qualitative assessment protocol used to determine ecosystem condition based on benchmark characteristics described in the Reference Sheet. A suite of 17 (or more) indicators are typically considered in an assessment. The ecological site(s) representative of an assessment location must be known prior to applying the protocol and must be verified based on soils and climate. Current plant community cannot be used to identify the ecological site.
| Author(s)/participant(s) | GK BRACKLEY |
|---|---|
| Contact for lead author | State Rangeland Management Specialist |
| Date | 06/20/2006 |
| Approved by | Kendra Moseley |
| Approval date | |
| Composition (Indicators 10 and 12) based on | Annual Production |
Indicators
-
Number and extent of rills:
Rills are typically non-existent. -
Presence of water flow patterns:
Water flow patterns are typically non-existent. Water flow patterns may rarely be observed on steeper slopes in areas recently subjected to summer convection storms or rapid spring snowmelt. -
Number and height of erosional pedestals or terracettes:
Pedestals are none to rare. Occurrence is usually limited to areas of water flow patterns. Frost heaving of shallow rooted plants should not be considered a "normal" condition. -
Bare ground from Ecological Site Description or other studies (rock, litter, lichen, moss, plant canopy are not bare ground):
Bare Ground 20-30% depending on amount of surface rock fragments. -
Number of gullies and erosion associated with gullies:
None -
Extent of wind scoured, blowouts and/or depositional areas:
None -
Amount of litter movement (describe size and distance expected to travel):
Fine litter (foliage from grasses and annual & perennial forbs) is expected to move the distance of slope length during intense summer convection storms or rapid snowmelt events. Persistent litter (large woody material) will remain in place except during large rainfall events. -
Soil surface (top few mm) resistance to erosion (stability values are averages - most sites will show a range of values):
Soil stability values should be 3 to 6 on most soil textures found on this site. (To be field tested.) -
Soil surface structure and SOM content (include type of structure and A-horizon color and thickness):
Surface structure is typically thin to thick platy, subangular blocky or massive. Soil surface colors are dark and the soils are typified by a mollic epipedon. Organic matter of the surface 2 to 4 inches is typically 1.25 to 3 percent. Organic matter content can be more or less depending on micro-topography. -
Effect of community phase composition (relative proportion of different functional groups) and spatial distribution on infiltration and runoff:
Perennial herbaceous plants (especially deep-rooted bunchgrasses [i.e., needlegrasses & basin wildrye]) slow runoff and increase infiltration. Shrub canopy and associated litter break raindrop impact and provide opportunity for snow catch and accumulation on site. -
Presence and thickness of compaction layer (usually none; describe soil profile features which may be mistaken for compaction on this site):
Compacted layers are none. Platy or massive sub-surface horizons or subsoil argillic horizons are not to be interpreted as compaction. -
Functional/Structural Groups (list in order of descending dominance by above-ground annual-production or live foliar cover using symbols: >>, >, = to indicate much greater than, greater than, and equal to):
Dominant:
Reference Plant Community: Deep-rooted, cool season, perennial bunchgrasses >> tall shrubs (big sagebrush & antelope bitterbrush). (By above ground production)Sub-dominant:
Associated shrubs > deep-rooted, cool season, perennial forbs = shallow-rooted, cool season, perennial grasses and grass-like plants > fibrous, shallow-rooted, cool season, perennial and annual forbs. (By above ground production)Other:
Evergreen treesAdditional:
-
Amount of plant mortality and decadence (include which functional groups are expected to show mortality or decadence):
Dead branches within individual shrubs may be common with standing dead shrub canopy material as much as 15% of total woody canopy; some of the mature bunchgrasses (<10%) have dead centers. -
Average percent litter cover (%) and depth ( in):
Between plant interspaces (30-45%) and litter depth is ± ½ inch. -
Expected annual annual-production (this is TOTAL above-ground annual-production, not just forage annual-production):
For normal or average growing season (through mid-June) ± 1500 lbs/ac; Spring moisture significantly affects total production. Favorable years ± 2000 lbs/ac and unfavorable years ± 1000 lbs/ac. -
Potential invasive (including noxious) species (native and non-native). List species which BOTH characterize degraded states and have the potential to become a dominant or co-dominant species on the ecological site if their future establishment and growth is not actively controlled by management interventions. Species that become dominant for only one to several years (e.g., short-term response to drought or wildfire) are not invasive plants. Note that unlike other indicators, we are describing what is NOT expected in the reference state for the ecological site:
Potential nvaders on this site include cheatgrass, annual mustards, and knapweeds. Rabbitbrush, Utah juniper, and singleleaf pinyon are increasers on this site. -
Perennial plant reproductive capability:
All functional groups should reproduce in average (or normal) and above average growing season years. Little growth or reproduction occurs in drought years.
Print Options
Sections
Font
Other
The Ecosystem Dynamics Interpretive Tool is an information system framework developed by the USDA-ARS Jornada Experimental Range, USDA Natural Resources Conservation Service, and New Mexico State University.
Click on box and path labels to scroll to the respective text.