Ecological site group R023XY902OR
Shallow and Moderately Deep >12 PZ Low and Lahontan sagebrush and Idaho fescue
Last updated: 06/03/2024
Accessed: 02/21/2026
Ecological site group description
Key Characteristics
- Site does not pond or flood
- Landform other than dunes
- Soil surface is clayey
- MAP > 10"
- Soil Temperature Regime Frigid. Frost Free Days per Year < 80
Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site.
Physiography
This group is on plateaus and mountains above 5,500 feet. Slopes are typically 2 to 20 percent. Slopes up to 75 percent are uncommon.
Climate
The climate is classified as Cold Semi-Arid in the Koppen Classification System.
The area receives between 12 and 20 inches of annual precipitation as snow in the winter and rain in spring and fall. Summers are generally dry.
The frost-free period is 50 to 90 days. The mean annual air temperature is between 40 and 45 °F.
Soil features
The soils in this group have bedrock or another root restrictive layer within 36 inches of the surface. The textures are clayey and loamy-skeletal.
The soil temperature regime is frigid. The soils are principally Mollisols. There are some Aridisols on R023XY090NV. Common soil series in this ecological site group include Ninemile and Hutchley.
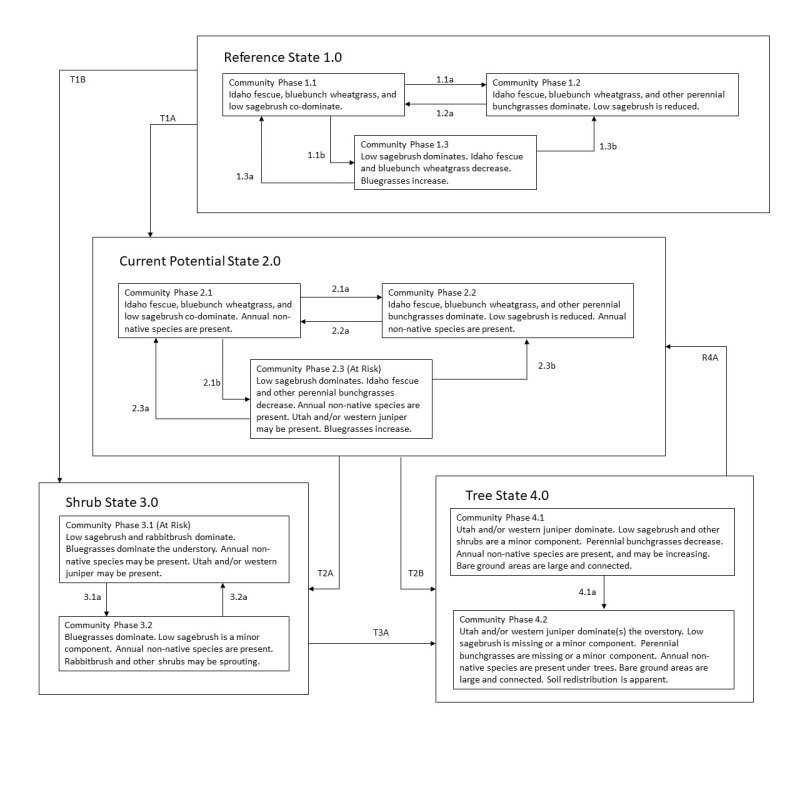
Vegetation dynamics
Ecological Dynamics and Disturbance Response:
An ecological site is the product of all the environmental factors responsible for its development. Each site has a set of key characteristics that influence its resilience to disturbance and resistance to invasives. According to Caudle et al. (2013), key characteristics include:
1. Climate factors such as precipitation and temperature.
2. Topographic characteristics such as aspect, slope, elevation, and landform.
3. Hydrologic processes such as infiltration and runoff.
4. Soil characteristics such as depth, texture, structure, and organic matter.
5. Plant communities and their functional groups and productivity.
6. Natural disturbance (fire, herbivory, etc.) regime.
Biotic factors that influence resilience include site productivity, species composition and structure, and population regulation and regeneration (Chambers et al., 2013).
The ecological sites in this group are dominated by deep-rooted, cool-season, perennial bunchgrasses and long-lived shrubs (at least 50 years old) with high root to shoot ratios. The dominant shrubs usually root to the full depth of the winter-spring soil moisture recharge, which ranges from 1.0 to over 3.0 meters (Dobrowolski et al., 1990). However, community types with low sagebrush (Artemisia arbuscula) as the dominant shrub may only have available rooting depths of 71 to 81 centimeters (Jensen, 1990). These shrubs have a flexible generalized root system with development of both deep taproots and laterals near the surface (Comstock & Ehleringer, 1992).
Periodic drought regularly influences sagebrush ecosystems, and drought duration and severity have increased throughout the 20th century in much of the Intermountain West. Major shifts away from historical precipitation patterns have the greatest potential to alter ecosystem function and productivity. Species composition and productivity can be altered by the timing of precipitation and water availability within the soil profile (Bates et al., 2006).
Low sagebrush is fairly drought tolerant but also tolerates periodic wetness during some portion of the growing season (Fosberg & Hironaka, 1964; Blackburn et al., 1968a, 1968b, 1969). It grows on soils that have a strongly structured B2t (argillic) horizon close to the soil surface (Winward, 1980; Fosberg & Hironaka, 1964; Zamora & Tueller, 1973). Low sagebrush is also susceptible to the sagebrush defoliator known as the Aroga moth (Aroga websteri). While the Aroga moth can partially or entirely kill individual plants or entire stands of big sagebrush (Artemisia tridentata) (Furniss & Barr, 1975), research is inconclusive of the damage sustained by low sagebrush populations.
Lahontan sagebrush was only recently identified as a unique species of sagebrush (Winward & McArthur, 1995). Lahontan sagebrush is a cross between low sagebrush and Wyoming sagebrush (Artemisia tridentata ssp. wyomingensis). It typically grows near the old shorelines of Lake Lahontan from the Pleistocene Epoch. This subspecies grows on soils similar to low sagebrush with shallow depths and low water holding capabilities (Winward& McArthur, 1995).
In the Clay Plain ecological site, early sagebrush (Artemisia arbuscula ssp. longiloba) is the dominant shrub. Early sagebrush (also known as alkali sagebrush) is a unique subspecies of Artemisia arbuscula that is differentiated because it blooms in mid-June to July. It was originally named alkali sagebrush because it was found on alkaline limestone soils (Beetle, 1960). However, a body of research challenges this claim across the species’ range (Passey & Hugie, 1962; Robertson et al., 1966; Zamora & Tueller, 1973). It grows on soils similar to low sagebrush, with a restrictive horizon close to the soil surface (Robertson et al., 1966; Zamora & Tueller, 1973).
The Great Basin sagebrush communities have high spatial and temporal variability in precipitation both among years and within growing seasons (MacMahon, 1980). Nutrient availability is typically low but increases with elevation and closely follows moisture availability. The invasibility of plant communities is often linked to resource availability. Disturbance changes resource uptake and increases nutrient availability, often to the benefit of non-native species; native species are often damaged and their ability to use resources is depressed for a time, but resource pools may increase from lack of use and/or the decomposition of dead plant material following disturbance (Whisenant, 1999; Miller et al., 2013). The invasion of sagebrush communities by cheatgrass (Bromus tectorum) has been linked to disturbances that result in fluctuations in resources such as fire and abusive grazing (Beckstead & Augspurger, 2004; Chambers et al., 2007; Johnson et al., 2011).
The ecological sites in this group have moderate to high resilience to disturbance and resistance to invasion. Resilience increases with elevation, northerly aspect, precipitation, and nutrient availability. Four possible stable states have been identified for this group.
Fire Ecology:
Low sagebrush is killed by fire and does not resprout (Tisdale & Hironaka, 1981). Fire risk is greatest following a wet, productive year when there is greater production of fine fuels (Beardall & Sylvester, 1976). Fire return intervals are not well understood because these ecosystems rarely coincide with fire-scarred conifers, but a wide range of 20 to well over 100 years has been estimated (Miller & Rose, 1995, 1999; Baker, 2006; Knick et al., 2005).
Historically, fires were probably patchy due to the low productivity of these sites (Beardall & Sylvester, 1976; Ralphs & Busby, 1979; Wright et al., 1979; Smith & Busby, 1981). Fine fuel loads generally average 100 to 400 pounds per acre (110 to 450 kilograms per hectare) but are occasionally as high as 600 pounds per acre (680 kilograms per hectare) in low sagebrush habitat types (Bradley et al., 1992). Reestablishment occurs from off-site wind-dispersed seed (Young, 1983). Recovery time of low sagebrush following fire is variable (Young, 1983). After fire, if regeneration conditions are favorable, low sagebrush recovers in 2 to 5 years; on harsh sites where cover is low to begin with and/or erosion occurs after fire, recovery may require more than 10 years (Young, 1983). Slow regeneration may subsequently worsen erosion (Blaisdell et al., 1982). We were unable to find any substantial research on success of seeding low sagebrush after fire. To date, we have not been able to find specific research on the fire response of Lahontan sagebrush.
The effect of fire on bunchgrasses relates to culm density, culm-leaf morphology, and the size of the plant. Idaho fescue (Festuca idahoensis) is the dominant grass on these communities. Idaho fescue’s response to fire varies with condition and size of the plant, season and severity of fire, and ecological conditions. Mature Idaho fescue plants are commonly severely damaged by fire in all seasons (Wright et al., 1979). Rapid burns leave little damage to root crowns, and production of new tillers corresponds with the onset of fall moisture (Johnson et al., 1994). However, Wright et al. (1979) found the dense, fine leaves of Idaho fescue provided enough fuel to burn for hours after a fire had passed, thereby killing or seriously injuring the plant regardless of the intensity of the fire. Idaho fescue is generally more sensitive to fire than the other prominent grasses on these sites such as bluebunch wheatgrass (Pseudoroegneria spicata) (Conrad & Poulton, 1966). However, Robberecht and Defossé (1995) suggest the latter is more sensitive. They observed culm and biomass reduction of bluebunch wheatgrass following fires of moderate severity, whereas Idaho fescue required high fire severity for a similar reduction in culm and biomass. Also, given the same fire severity treatment, post-fire culm production initiated earlier and more rapidly in Idaho fescue.
Bluebunch wheatgrass has coarse stems with little leafy material; therefore, the plant’s aboveground biomass burns rapidly and little heat is transferred downward into the crowns (Young, 1983). Bluebunch wheatgrass is typically fairly tolerant of burning, except in May in eastern Oregon (Britton et al., 1990). Uresk et al. (1976) reported burning increased vegetative and reproductive vigor of bluebunch wheatgrass. Bluebunch wheatgrass experiences slight damage from fire but is more susceptible to fire damage in drought years (Young, 1983). Most authors classify the plant as undamaged by fire (Kuntz, 1982).
Thurber’s needlegrass (Achnatherum thurberianum), a minor component on these sites, is very susceptible to fire-caused mortality. Burning can decrease the vegetative and reproductive vigor of Thurber’s needlegrass (Uresk et al., 1976). Fire can cause high mortality in addition to reducing basal area and yield of Thurber’s needlegrass (Britton et al., 1990). The fine leaves and densely tufted growth form make this grass susceptible to subsurface charring of the crowns (Wright & Klemmedson, 1965). Although timing of fire highly influences the response and mortality of Thurber’s needlegrass, smaller bunch sizes are less likely to be damaged by fire (Wright & Klemmedson, 1965). However, Thurber’s needlegrass often survives fire and will continue growth when conditions are favorable (Koniak, 1985). Thus, the initial condition of the bunchgrasses on the site and seasonality and intensity of the fire all factor into the individual species response.
Sandberg bluegrass (Poa secunda), a minor component on these ecological sites, can increase following fire likely due to its low stature and productivity (Daubenmire, 1975) and may slow reestablishment of more deeply rooted bunchgrasses.
Livestock/Wildlife Grazing Interpretations:
Domestic sheep and, to a much lesser degree, cattle consume low sagebrush, particularly during the spring, fall, and winter (Sheehy & Winward, 1981). Heavy dormant season grazing by sheep will reduce sagebrush cover and increase grass production (Laycock, 1967). Trampling damage, particularly from cattle or horses, in low sagebrush habitat types is greatest on areas with highly clayey soils during spring snowmelt when surface soils are saturated. In drier areas with more gravelly soils, trampling is less of a problem (Hironaka et al., 1983). Bunchgrasses, in general, best tolerate light grazing after seed formation. Britton et al. (1990) observed the effects of clipping date on basal area of five bunchgrasses in eastern Oregon and found grazing from August to October (after seed set) has the least impact.
Heavy grazing during the growing season will reduce perennial bunchgrasses and increase sagebrush (Laycock, 1967). Abusive grazing by cattle or horses allows unpalatable plants like low sagebrush, rabbitbrush, and some forbs such as arrowleaf balsamroot (Balsamorhiza sagittata) to become dominant on the site. Sandberg bluegrass is grazing tolerant due to its short stature. Annual, non-native, weedy species such as cheatgrass, mustards, and medusahead may invade.
Throughout 2 years of site visits, Lahontan sagebrush was observed in a heavily-browsed state on several ecological sites in this group. This recently differentiated subspecies of low sagebrush (Winward & McArthur, 1995) is moderately to highly palatable to browse species (McArthur, 2005; Rosentreter, 2005). Dwarf sagebrush species such as Lahontan sagebrush, low sagebrush, and black sagebrush are preferred by mule deer for browse among the sagebrush species.
Idaho fescue tolerates light to moderate grazing (Ganskopp & Bedell, 1981). It is moderately resistant to trampling (Cole, 1987). Heavy grazing may lead to replacement of Idaho fescue with non-native species such as cheatgrass (Mueggler, 1975).
Bluebunch wheatgrass is moderately grazing tolerant and is very sensitive to defoliation during the active growth period (Blaisdell & Pechanec, 1949; Laycock, 1967; Anderson & Scherzinger, 1975; Britton et al., 1990). In studies, herbage and flower stalk production were reduced with clipping at all times during the growing season; clipping was most harmful, however, during the boot stage (Blaisdell & Pechanec, 1949; Britton et al., 1990). Tiller production and growth of bluebunch wheatgrass can be greatly reduced when clipping is coupled with drought (Busso & Richards, 1995). Mueggler (1975) estimated that low-vigor bluebunch wheatgrass may need up to 8 years rest to recover.
Thurber’s needlegrass is an important forage source for livestock and wildlife in the arid regions of the West (Ganskopp, 1988). The seeds (despite their hard callus) are apparently not injurious, but grazing animals avoid them when the seeds begin to mature. Sheep, however, have been observed grazing the leaves closely, leaving stems untouched (Eckert & Spencer, 1987). Heavy grazing during the growing season can reduce the basal area of Thurber’s needlegrass (Eckert & Spencer, 1987). A single defoliation, particularly during the boot stage, can reduce herbage production and root mass thus potentially lowering the competitive ability of this needlegrass (Ganskopp, 1988).
Reduced bunchgrass vigor or density provides an opportunity for Sandberg bluegrass and/or cheatgrass and other invasive species to expand onto or occupy interspaces. Sandberg bluegrass increases under grazing pressure (Tisdale & Hironaka, 1981). It is capable of co-existing with cheatgrass or other weedy species. Excessive sheep grazing favors Sandberg bluegrass; however, where cattle are the dominant grazers, cheatgrass often dominates (Daubenmire, 1970). Thus, depending on the season of use, the type of grazing animal, and site conditions, either Sandberg bluegrass or cheatgrass may become the dominant understory species with inappropriate grazing management.
References:
Beardall, L. E. and V. E. Sylvester. 1976. Spring burning of removal of sagebrush competition in Nevada. In: Tall Timbers fire ecology conference and proceedings. Tall Timbers Research Station. 14:539-547.
Beetle, A. A. 1960. A Study of Sagebrush: The Section Tridentatae of Artemisia. Wyoming Agricultural State Bulletin 368. 83 p.
Beckstead, J., and Augspurger, C. K. 2004. An experimental test of resistance to cheatgrass invasion: limiting resources at different life stages. Biological Invasions 6(4):417-432.
Blackburn, W. H., P. T. Tueller, and R. E. Eckert, Jr. 1968a. Vegetation and soils of the Duckwater Watershed. Agr. Exp. Sta., Univ. of Nev., R40.
Blackburn, W. H., P. T. Tueller, and R. E. Eckert, Jr..1968b. Vegetation and soils of the Crowley Creek Watershed. Agr. Exp. Sta., Univ. of Nev., R42.
Blackburn, W. H., P. T. Tueller, and R. E. Eckert, Jr., 1969a. Vegetation and soils of the Churchill Canyon Watershed. Agr. Exp. Sta., Univ. of Nev., R45.
Blaisdell, J. P. and J. F. Pechanec. 1949. Effects of Herbage Removal at Various Dates on Vigor of Bluebunch Wheatgrass and Arrowleaf Balsamroot. Ecology 30(3):298-305.
Blaisdell, J. P., R. B. Murray, and E. D. McArthur. 1982. Managing intermountain rangelands-sagebrush-grass ranges. Gen. Tech. Rep. INT-134. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station, Ogden, UT. 41 p.
Bradley, A. F., N. V. Noste, and W. C. Fischer. 1992. Fire ecology of forests and woodlands in Utah. Gen. Tech. Rep. INT-287. U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 128 p.
Britton, C. M., G. R. McPherson, and F. A. Sneva. 1990. Effects of burning and clipping on five bunchgrasses in eastern Oregon. Great Basin Naturalist 50(2):115-120.
Comstock, J. P. and J. R. Ehleringer. 1992. Plant adaptation in the Great Basin and Colorado plateau. Western North American Naturalist 52(3):195-215.
Daubenmire, R. 1970. Steppe vegetation of Washington. Technical Bulletin 62. Washington Agriculture Experiment Station. 131 p.
Daubenmire, R. 1975. Plant succession on abandoned fields, and fire influences in a steppe area in southeastern Washington. Northwest Science 49(1):36-48.
Dobrowolski, J. P., M. M. Caldwell, and J. H. Richards. 1990. Basin hydrology and plant root systems.Pages 243-292 in C. B. Osmond, L. F. Pitelka, and G. M. Hidy (eds.). Plant biology of the basin and range. Springer-Verlag, New York.
Fosberg, M. A., and M. Hironaka. 1964. Soil properties affecting the distribution of big and low sagebrush communities in southern Idaho. American Society of Agronomy Special Publication No. 5. Pages 230-236.
Furniss, M. M. and W. F. Barr. 1975. Insects affecting important native shrubs of the northwestern United States Gen. Tech. Rep. INT-19. Intermountain Forest and Range Experiment Station, U.S. Department of Agriculture, Forest Service. Ogden, UT. 68 p.
Ganskopp, D. C., and T. E. Bedell. 1981. An assessment of vigor and production of range grasses following drought. Journal of Range Management 34(2):137-141.
Hironaka, M., M. A. Fosberg, and A. H. Winward. 1983. Sagebrush-grass habitat types of southern Idaho. Bulletin Number 35. University of Idaho, Forest, Wildlife and Range Experiment Station, Moscow, ID.
Johnson, B. G.; Johnson, D. W.; Chambers, J. C.; Blank, B. R. 2011. Fire effects on the mobilization and uptake of nitrogen by cheatgrass (Bromus tectorum L.). Plant and Soil 341(1-2):437-445.
Johnson, C. G., Jr., R. R. Clausnitzer, P. J. Mehringer, and C. Oliver. 1994. Biotic and abiotic processes of Eastside ecosystems: the effects of management on plant and community ecology and on stand and landscape vegetation dynamics. Gen. Tech. Rep. PNW-GTR-322. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 66 p.
Knick, S. T., Holmes, A. L. and Miller, R. F. 2005. The role of fire in structuring sagebrush habitats and bird communities. Studies in Avian Biology 30:63-75.
Koniak, S. 1985. Succession in pinyon-juniper woodlands following wildfire in the Great Basin. The Great Basin Naturalist 45(3):556-566.
Kuntz, D. E. 1982. Plant response following spring burning in an Artemisia tridentata subsp. vaseyana/Festuca idahoensis habitat type. Moscow, ID: University of Idaho. 73 p. Thesis.
MacMahon, J. A. 1980. Ecosystems over time: succession and other types of change. In: Waring, R., ed. Proceedings—Forests: fresh perspectives from ecosystem analyses. Biological Colloquium. Corvallis, OR: Oregon State University. Pages 27-58.
McArthur, E. 2005. View Points: Sagebrush, Common and Uncommon, Palatable and Unpalatable Rangelands 27(4):47-51.
Miller, R. F., and J. A. Rose. 1995. Historic expansion of Juniperus occidentalis (western juniper) in southeastern Oregon. Western North American Naturalist 55(1):37-45.
Miller, R. F., Chambers, J. C., Pyke, D. A., Pierson, F. B. and Williams, C. J., 2013. A review of fire effects on vegetation and soils in the Great Basin Region: response and ecological site characteristics. Gen. Tech. Rep. RMRS-GTR-308. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. 126 p.
Passey, H. B., and V. K. Hugie. 1962. Sagebrush on relict ranges in the Snake River plains and northern Great Basin. Journal of Range Management 15(5):273-278.
Ralphs, M. H., and F. E. Busby. 1979. Prescribed burning: vegetative change, forage production, cost, and returns on six demonstration burns in Utah. Journal of Range Management 32(4):267–270.
Robertson, D. R., J. L. Nielsen, and N. H. Bare. 1966. Vegetation and Soils of Alkali Sagebrush and Adjacent Big Sagebrush Ranges in North Park, Colorado. Journal of Range Management 19(1):17-20.
Rosentreter, R. 2005. Sagebrush Identification, Ecology, and Palatability Realtive to Sage Grouse. In: Shaw, Nancy L.; Pellant, Mike; Monsen, Stephen B., (comps.). Sage-grouse habitat restoration symposium proceedings; 2001 June 4-7, Boise, ID. Proc. RMRS-P-38. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. Vol. 38: 3-16.
Smith, M. A., and F. Busby. 1981. Prescribed burning, effective control of sagebrush in Wyoming. Agricultural Experiment Station. RJ-165. University of Wyoming, Laramie, Wyoming, USA. 12 p.
Tisdale, E. W. and M. Hironaka. 1981. The sagebrush-grass region: A review of the ecological literature. Bulletin 33. University of Idaho, Forest, Wildlife and Range Experiment Station. Moscow, ID. 31 p.
Whisenant, S., 1999. Repairing Damaged Wildlands: a process-orientated, landscape-scale approach (Vol. 1). Cambridge, UK: Cambridge University Press. 312 p.
Winward, A. H. 1980. Taxonomy and ecology of sagebrush in Oregon. Station Bulletin 642. Oregon State University Agricultural Experiment Station. Corvallis, OR. 15 p.
Winward, A. H., and E. D. McArthur. 1995. Lahontan sagebrush (Artemisia arbuscula ssp. longicaulis): a new taxon. Great Basin Naturalist 55(2):151-157.
Wright, H. A., L. F. Neuenschwander, and C. M. Britton. 1979. The role and use of fire in sagebrush-grass and pinyon-juniper plant communities: A state-of-the-art review. Gen. Tech. Rep. INT-58. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 48 p.
Young, R. P. 1983. Fire as a vegetation management tool in rangelands of the Intermountain region. In: Monsen, S.B. and N. Shaw (eds). Managing Intermountain rangelands—improvement of range and wildlife habitats: Proceedings. 1981, September 15-17; Twin Falls, ID; 1982, June 22-24; Elko, NV. Gen. Tech. Rep. INT-157. Ogden, UT. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. Pages 18-31.
Zamora, B., and Tueller, P. T. 1973. Artemisia arbuscula, A. longiloba, and A. nova habitat types in northern Nevada. Great Basin Naturalist 33(4):225-242
Major Land Resource Area
MLRA 023X
Malheur High Plateau
Subclasses
- R023XY008NV–MOUNTAIN RIDGE
- R023XY014NV–SHALLOW LOAM 14+ P.Z.
- R023XY017NV–CLAYPAN 14-16 P.Z.
- R023XY090NV–CLAY PLAIN
- R023XY211OR–PUMICE CLAYPAN 10-12 PZ
- R023XY216OR–CLAYPAN 12-16 PZ
- R023XY217OR–JUNIPER TABLELAND 12-16 PZ
- R023XY312OR–SHALLOW NORTH 12-16 PZ
- R023XY410OR–GRAVELLY RIDGE 12-16 PZ
- R023XY412OR–GRAVELLY RIDGE 16+ PZ
- R023XY507OR–CLAYPAN 16-25 PZ
- R023XY511OR–JUNIPER LAVA BENCHES 9-12 PZ
Correlated Map Unit Components
22177325, 22177348, 22177349, 22177110, 22177113, 22177228, 22177475, 22177230, 22177476, 22177233, 22177123, 22177127, 22177583, 22177584, 22177586, 22177587, 22177538, 21500867, 21500861, 21501522, 21501014, 21501141, 21501195, 21500993, 21501257, 21501263, 21501236, 21501235, 21501181, 21501516, 21501519, 21500731, 21501551, 21500713, 21501305, 21501308, 21501540, 21500854, 21501246, 21500941, 21500958, 21500903, 21501311, 21582072, 21582565, 21582570, 21589867, 21590076, 21589827, 21590047, 21589899, 21589883, 21590063, 21589468, 21589394, 21589962, 21589804, 21589678, 21589504, 21589653, 21589836, 21589438, 21589765, 21589762, 21589593, 21589641, 21590004, 21590005, 21589629, 21589630, 21589638, 21589637, 21590000, 21589833, 21589596, 21589598, 21589853, 21589950, 21590289, 21590855, 21590756, 21590545, 21590717, 21590368, 21590869, 21590375, 21590710, 21590561, 21590926, 21590913, 21590667, 21590311, 21590567, 21590575, 21590675, 21590830, 21604715, 21604135, 21604286, 21604612, 21605186, 21604599, 21605197, 21604406, 21729121, 21729123, 21729153, 21729169, 21729141, 21728950, 21728759, 21729307, 21730061, 21729524, 21729527, 21729513, 21729519, 21728975, 21730107, 21729571, 21729009, 21729014, 21729020, 22168434, 22168432, 22168433, 22168242, 22168240, 22168247, 22168268, 22168271, 22168272, 22168234, 22168260, 22168251, 22168267, 22168263, 22168264, 22168252, 22168253, 22168228, 22168227, 22168225, 22168255, 22168261, 22168262, 22170526, 22170523, 22170520, 22170491, 22171257, 22171254, 22171258, 22171261, 22171243, 22171250, 22171237, 22171218, 22171185, 22171182, 22171176, 22171164, 22171155, 22171135, 22171129, 22170999, 22170982, 22170979, 22170465, 22170581, 22170900, 22170901, 22170929, 22170927, 22170925, 22170921, 22170917, 22170914, 22170912, 22170908, 22170905, 22170902, 22170903, 22170896, 22170851, 22170846, 22170847, 22170611, 22176890, 22176680, 22176880, 22175176, 22176487, 22176790, 22175764, 22175448, 22176799, 22176820, 22175038, 22175053, 22175033, 22176856, 22175088, 22175690, 22175694, 22175695, 22175776, 22175112, 22175753, 22176753, 22176754, 22176380, 22176331, 22175073, 22176950, 22175615, 22175551, 22175024, 22175482, 22175773, 22176571, 22175627, 22175547, 22175102, 22175808, 22175809, 22175583, 22175584, 22175485, 22175486, 22175167, 22175191, 22176291, 22176502, 22176918, 22175163, 22175154, 22176398, 22176301, 22176281, 22176283, 22176642, 22176298, 22176287, 22175827, 22175653, 22176833, 22175490, 22175716, 22175823, 22175061, 22175554, 22175712, 22175630, 22175631, 22175559, 22175560, 22175568, 22175122, 22175141, 22177264, 22177284, 22177285, 22177286, 22177159, 22177175, 22177181, 22177068, 22177069, 22177079, 22177080, 22177077, 22177078, 22177193, 22177194, 22177445, 22177444, 22177323, 22177324
Stage
Provisional
Contributors
T Stringham (UNR under contract with BLM)
DMP
Click on box and path labels to scroll to the respective text.